Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Moderate disease in rheumatoid arthritis (RA) is defined as a 28-joint disease activity score (DAS28) of 3.2-5.1(MDAS). As per the National Institute for Health and Care Excellence (NICE, TA 715, TA 744 & TA 676), patients with moderate RA failing ≥2 conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) should be assessed for eligibility for a TNF-inhibitor, upadacitinib or filgotinib [1].We aimed to assess compliance with NICE guidance on moderate RA management in a diverse area of London.
Methods: All patients with confirmed RA (EULAR/ACR 2010 criteria [2]) seen in the last 18 months were extracted from the electronic records (Epic) of King’s College Hospital NHS Foundation Trust. Patients with recorded MDAS and previously receiving ≥2 csDMARDs were included. Exclusion criteria were: temporarily stopping advanced therapy (eg due to malignancy or infection); commencing a biologic or targeted synthetic DMARD (b/tsDMARD) not specified in TA 715, 744 or 676; no recorded DAS28 or components.Patients were analysed in two groups:i) those on a biologic via the moderate RA pathway;ii) those not on a biologic. All patients in group I were automatically eligible for inclusion. For group II, a random sample of 250 patients were reviewed in depth for encounters in which MDAS was recorded. If MDAS was present, reasons for not starting advanced therapy were extracted from Epic, if available. Other data extracted included demographics, DAS28, comorbidities and treatment history.Results were summarised using descriptive statistics. Reasons for delaying therapy were summarised using narrative synthesis.
Results: In total, 87 patients commenced advanced therapy via the moderate RA pathway (mean age [SD]: 53 [14.7]; 55% female), most commonly adalimumab (n=70) (Table 1).Of the 250 patients not on a b/tsDMARD, 20 had no recorded DAS (5% (n=13) did not attend a rheumatology appointment; 3% (n=7) had telephone encounters). 2% (n=6) had MDAS documented on ≥1 occasions (3.23-4.23), 50% female and mean age 63 (SD 8.0).In all six MDAS cases, no offer of advanced therapy was recorded. One patient with MDAS had “apprehension about starting new medications” due to previous DMARD side-effects. Three patients with MDAS were subsequently assessed via telephone, precluding DAS assessment (Figure 1).
Conclusion: Our results demonstrate good compliance with NICE on escalation to advanced therapies in patients with MDAS. Increased telehealth usage limits assessment of joints and suitability for treatment escalation, especially in district hospitals. Self-assessment tools, to complete prior to telephone appointments, may mitigate this. Clinicians may be reluctant to discuss advanced therapies if patients demonstrate medication hesitancy. Shared decision-making, appropriate triage for telehealth assessment and greater use of self-assessment tools are vital in facilitating discussions and commencement of advanced therapy in moderate RA.References1. National Institute for Health and Care Excellence. 2021. TA715, TA744 & TA676. Available at https://www.nice.org.uk/. 2. Aletaha, D et al. 2010 Rheumatoid arthritis classification criteria: An ACR/EULAR collaborative initiative. Arthritis & Rheumatism.
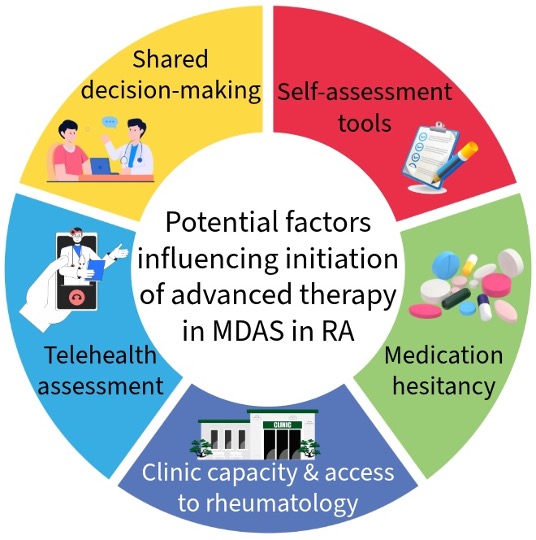 Table 1: Summary of characteristics of included patients with rheumatoid arthritis (RA), including those commenced on advanced therapy via the moderate RA pathway during the 18-month study period.
Table 1: Summary of characteristics of included patients with rheumatoid arthritis (RA), including those commenced on advanced therapy via the moderate RA pathway during the 18-month study period.
.jpg) Figure 1: Summary of potential reasons for commencing or delaying advanced therapy in the setting of moderate disease activity (MDAS) in people with rheumatoid arthritis (RA). Possible reasons identified from this study include medication hesitancy (due to previous side-effects), telehealth assessments with no disease activity scores completed, and delays in accessing clinic appointments especially in rural or district hospitals. Mitigating factors may include the use of self-assessment tools in conjunction with telehealth and increased shared decision-making.
Figure 1: Summary of potential reasons for commencing or delaying advanced therapy in the setting of moderate disease activity (MDAS) in people with rheumatoid arthritis (RA). Possible reasons identified from this study include medication hesitancy (due to previous side-effects), telehealth assessments with no disease activity scores completed, and delays in accessing clinic appointments especially in rural or district hospitals. Mitigating factors may include the use of self-assessment tools in conjunction with telehealth and increased shared decision-making.
To cite this abstract in AMA style:
Kelly A, Rupnik M, Ahmed N, Dey M, Nikiphorou E. Advanced therapy use in RA patients with moderate disease activity in a large NHS Foundation Trust in South London, UK [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/advanced-therapy-use-in-ra-patients-with-moderate-disease-activity-in-a-large-nhs-foundation-trust-in-south-london-uk/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/advanced-therapy-use-in-ra-patients-with-moderate-disease-activity-in-a-large-nhs-foundation-trust-in-south-london-uk/
