Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Src homology region 2 domain-containing phosphatase 1 (SHP-1) is a cytoplasmic protein tyrosine phosphatase expressed in hematopoietic cells (PMID9069265). SHP-1 is a key negative regulator of adaptive and innate immune cell signaling; its function is critical in preventing and eliminating excessive immune cell activation (PMID12490957). Suboptimal SHP-1 function has been linked to autoimmune and inflammatory diseases (PMID27216862). We reported that genetically and pharmacologically enhanced SHP-1 activity reduced disease severity in a T cell-driven murine model of inflammatory arthritis (PMID32586377). Our objective was to determine if the protective role of enhanced SHP-1 activity is mediated by T cells and the adaptive immunity.
Methods: Proteoglycan-induced arthritis (PGIA) was induced in 12-weeks old female wildtype (WT) and Shp1- overexpressing transgenic (Shp1-Tg) mice. For adoptive cell transfer, unseparated spleen cells, regulatory T cells or conventional T cells were isolated from WT or Shp1-Tg mice and transferred to 12-weeks old female mice with Severe Combined Immunodeficiency (SCID). Collagen antibody-induced arthritis (CAIA) was induced in 12-weeks old female WT and Shp1-Tg mice by an antibody cocktail to collagen II. SHP-1 activity was enhanced by regorafenib (REG) treatment of WT mice with CAIA (20 mg/kg/day). Arthritis incidence and severity were determined by Visual Arthritis Score (VAS). Spleens were harvested to determine inflammatory cytokine gene expression by qPCR. Ankle joint histopathology was quantified. Animal experiments were approved by IACUC #23-059 at Rush University Medical Center. Data analysis software: GraphPad Prism v10.1.2. Statistical significance was set at p < 0.05.
Results: In the PGIA adoptive transfer model, SCID mice developed PGIA in each transfer group. PGIA severity in SCID mice was not statistically different following spleen cell transfer or T cell transfer from Shp1-Tg mice, indicating that the protective effect of genetically enhanced SHP-1 activity cannot be transferred by T (or B) cells (Fig 1A-B). In the CAIA model, Shp1-Tg mice showed significantly lower CAIA incidence and severity (Fig 1C) than WT mice. REG treatment of arthritic WT mice significantly reduced CAIA severity. REG treatment also significantly reduced expression of interleukin-1β, IL-6, IL-17 and interferon gamma transcripts. Ankle joint histopathology (synovial thickness, cartilage/bone damage, pannus, inflammatory cell infiltration) was significantly improved after REG treatment (Fig 1D-E).
Conclusion: Enhanced SHP-1 activity was protective/therapeutic in two different murine models of inflammatory arthritis. Since this protective effect was not transferred by spleen cells or T cells from Shp1-Tg mice, it is conceivable that innate immune cells (macrophages, neutrophils) are responsible for the anti-inflammatory effect of enhanced SHP-1 activity. Given the significant role of macrophages and neutrophils in RA, future studies will explore if enhanced SHP-1 activity in these cells can be utilized as a cell-based therapeutic approach in RA.
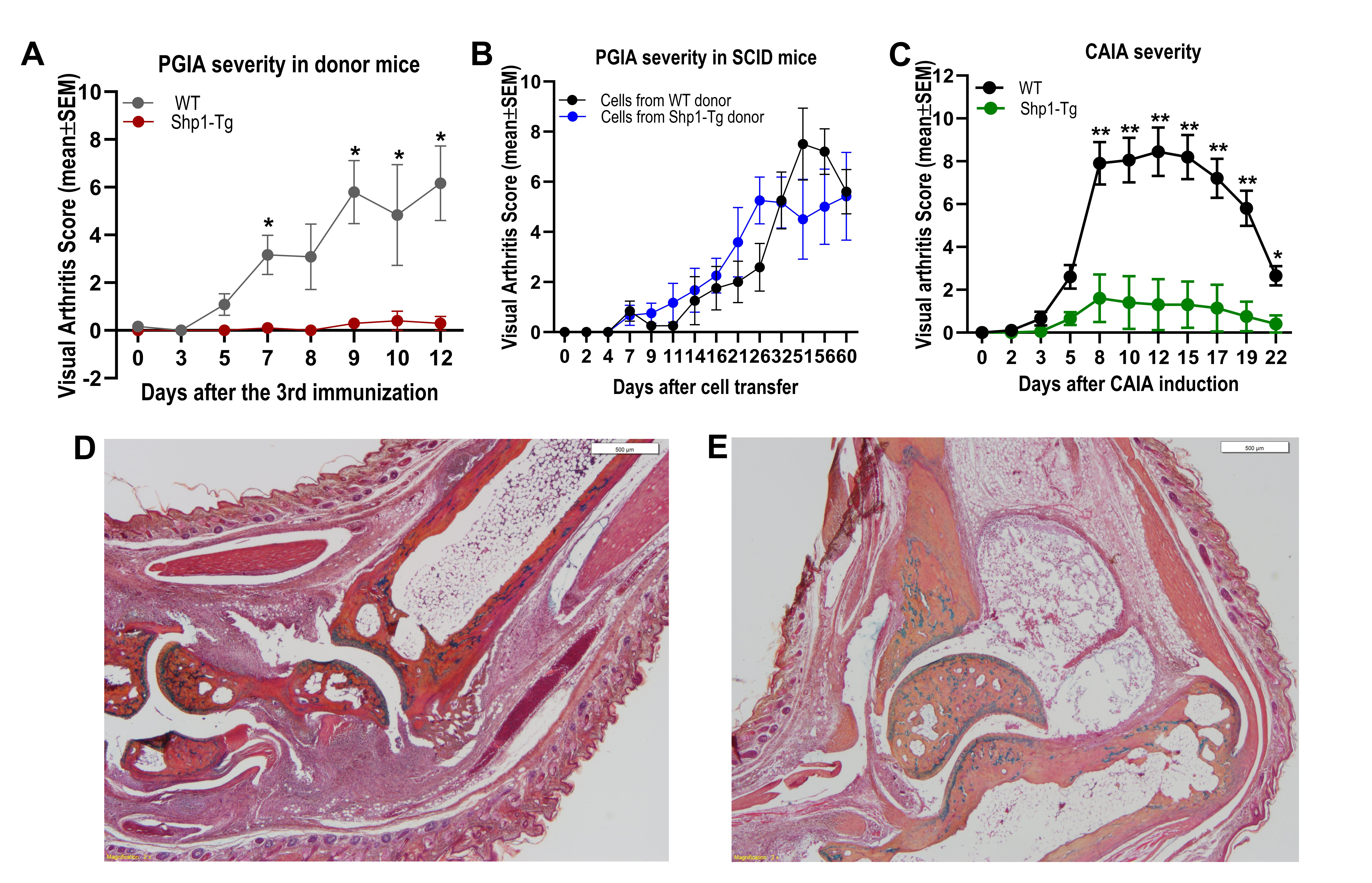 Figure 1. The anti-arthritic effect of SHP-1 overexpression is mediated by the innate immune system. A: Significantly reduced PGIA severity in spleen cell donor Shp1-Tg mice (12-weeks old female, n=10-12/group). B: No significant difference in PGIA severity in spleen cell recipient SCID mice (12-weeks old female, n=6-7/group). C: Shp1-Tg mice show significantly reduced CAIA severity compared to WT mice (n=10/group). D-E: Representative images of the tibiotarsal joint of WT mice with CAIA and treated with vehicle (D) or SHP-1 activator regorafenib (E). Alcian blue/hematoxylin/orange G staining. (p* < 0.05, p** < 0.01;two-way ANOVA followed by Sidak`s multiple comparison).
Figure 1. The anti-arthritic effect of SHP-1 overexpression is mediated by the innate immune system. A: Significantly reduced PGIA severity in spleen cell donor Shp1-Tg mice (12-weeks old female, n=10-12/group). B: No significant difference in PGIA severity in spleen cell recipient SCID mice (12-weeks old female, n=6-7/group). C: Shp1-Tg mice show significantly reduced CAIA severity compared to WT mice (n=10/group). D-E: Representative images of the tibiotarsal joint of WT mice with CAIA and treated with vehicle (D) or SHP-1 activator regorafenib (E). Alcian blue/hematoxylin/orange G staining. (p* < 0.05, p** < 0.01;two-way ANOVA followed by Sidak`s multiple comparison).
To cite this abstract in AMA style:
Li J, Mikecz K, Markovics A. Enhanced Src Homology Region 2 Domain-containing Phosphatase 1 Activity Ameliorates Murine Inflammatory Arthritis Through the Innate Immune System [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/enhanced-src-homology-region-2-domain-containing-phosphatase-1-activity-ameliorates-murine-inflammatory-arthritis-through-the-innate-immune-system/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/enhanced-src-homology-region-2-domain-containing-phosphatase-1-activity-ameliorates-murine-inflammatory-arthritis-through-the-innate-immune-system/
