Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Hydroxychloroquine (HCQ) is routinely prescribed for treatment of systemic lupus erythematosus (SLE) due to its efficacy at decreasing disease activity/SLE flares and strong benefit:risk ratio. However, the heterogeneity in the molecular mechanism of HCQ responses is not fully appreciated. The Study of Anti-Malarials in Incomplete Lupus Erythematosus (SMILE; NCT03030118), a double-blind, randomized, placebo-controlled trial to evaluate whether HCQ could delay or halt progression to SLE, provides longitudinal samples to characterize molecular differences in individuals who remained stable incomplete lupus erythematosus patients compared to those who gained additional criteria at some point during the 2-year study.
Methods: Baseline, 3 month, and 6 month serum samples from 105 Non-Progressors (did not gain SLICC criteria; 54 HCQ, 51 Placebo) and 54 Progressors (gained 1 or more SLICC criteria, 27 HCQ, 27 Placebo) from the SMILE trial were analyzed using the Olink Explore HT proteomics platform ( >5,000 proteins). After stringent quality control, analytes were retained only if the intra-individual coefficient of variation (CV) across multiple visits exceeded the technical CV in >90% of samples, resulting in 1,490 proteins for downstream analysis. Linear mixed effects models assessed protein changes over time by group. Proteins with nominal p < 0.2 were further analyzed by Gene Set Enrichment Analysis (GSEA) using Kyoto Encyclopedia of Genes and Genomes and Gene Ontology pathways.
Results: Proteomic analysis revealed both treatment- and progression-specific signatures. Analysis with GSEA found that both Non-Progressors and Progressors on HCQ showed downregulation of Toll-like receptor, TNF, IL-17 signaling pathways in addition to viral protein interaction pathways (Figure 1A), suggesting that these pathways represent HCQ effects independent of disease trajectory. Additionally, Non-Progressors on HCQ exhibited downregulation of JAK-STAT and MAPK signaling pathways while Progressors on Placebo show an increase in MAPK cascade over the first 6 months (Figure 1B). Progressors on Placebo also had increased inflammatory response pathways, while there was no pathway enrichment observed in Non-Progressors on placebo. IL-6 was uniquely decreased in Non-Progressors on HCQ (Figure 2A), while CCL3 was decreased and SOD2 was increased in Non-Progressors and Progressors on HCQ (Figure 2B). Non-Progressors on Placebo exhibited a decrease in CCL11, contrary to the increase seen in Non-Progressors on HCQ (Figure 2C). Progressors showed an increase in TNF over time (Figure 2D).
Conclusion: Within the first six months of treatment, HCQ exerts both disease-dependent and independent molecular effects, with distinct proteomic and pathway changes in individuals at risk for SLE progression. These findings illustrate the pleiotropic effect of HCQ and offer insight into pathways up-regulated in pre-clinical lupus.
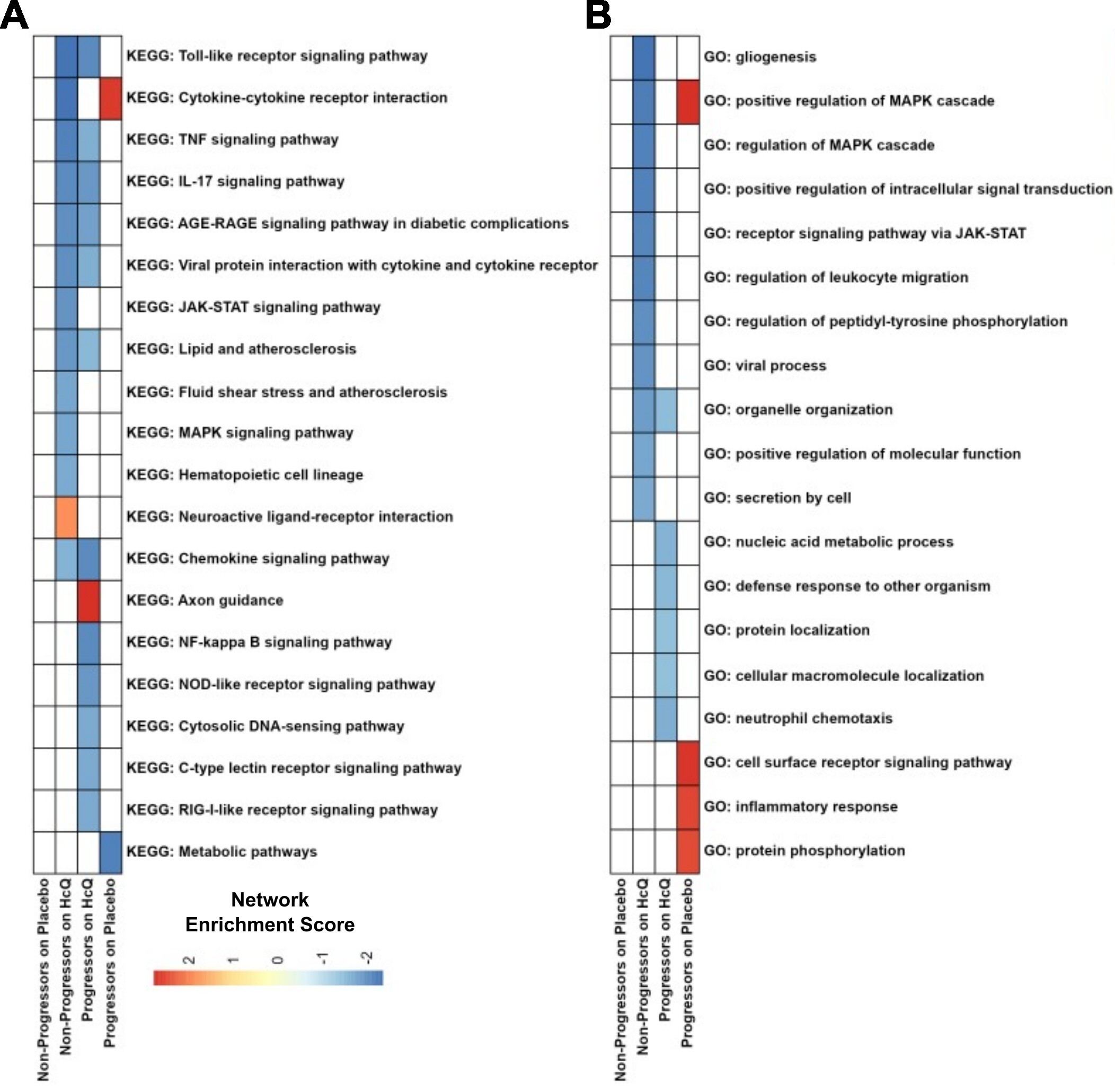 Figure 1. Pathway enrichment shows treatment and disease-specific signatures. Normalized enrichment scores (NES) for significantly enriched pathways (Q < 0.05), as identified by GSEA using Kyoto Encyclopedia of Genes and Genomes terms (A) and Gene Ontology Biological Process (B), comparing the first 6 months within each group.
Figure 1. Pathway enrichment shows treatment and disease-specific signatures. Normalized enrichment scores (NES) for significantly enriched pathways (Q < 0.05), as identified by GSEA using Kyoto Encyclopedia of Genes and Genomes terms (A) and Gene Ontology Biological Process (B), comparing the first 6 months within each group.
.jpg) Volcano plots showing changes in protein abundance between baseline and 6 months, as determined by linear mixed effects models in (A)Non-Progressors on HCQ, (B) Progressors on HCQ, (C) Non-Progressors on Placebo, and (D) Progressors on Placebo. X-axis indicates the fixed effect coefficient representing the change over time per stratified group, while legends indicate whether the protein was found to be significantly changed at P < 0.05 uniquely in the stratified group (red) or in multiple groups (blue).
Volcano plots showing changes in protein abundance between baseline and 6 months, as determined by linear mixed effects models in (A)Non-Progressors on HCQ, (B) Progressors on HCQ, (C) Non-Progressors on Placebo, and (D) Progressors on Placebo. X-axis indicates the fixed effect coefficient representing the change over time per stratified group, while legends indicate whether the protein was found to be significantly changed at P < 0.05 uniquely in the stratified group (red) or in multiple groups (blue).
To cite this abstract in AMA style:
Jones B, Smith M, Lu R, Guthridge C, Macwana S, DeJager W, Olsen N, Wagner C, James J, Karp D, Guthridge J. Longitudinal Proteomic Effects of Hydroxychloroquine in Individuals at Risk of Lupus: Differential Signatures in Progressors and Non-Progressors [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/longitudinal-proteomic-effects-of-hydroxychloroquine-in-individuals-at-risk-of-lupus-differential-signatures-in-progressors-and-non-progressors/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-proteomic-effects-of-hydroxychloroquine-in-individuals-at-risk-of-lupus-differential-signatures-in-progressors-and-non-progressors/
