Session Information
Date: Monday, November 18, 2024
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical II
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Myofibroblasts are key cells in the pathogenesis of systemic sclerosis (SSc). TGFβ is a key growth factor driving myofibroblast formation in SSc. The main downstream signaling pathways regulated by TGFβ during myofibroblast differentiation are less well understood. Various intracellular pathways play an important role in myofibroblast pathogenesis in SSc, such as the phosphatidylinositol 3-kinase/AKT serine/threonine kinase (PI3K/AKT) signaling pathway.
Antibody subsets in SSc are associated with distinct patterns of skin disease and organ manifestations. Recent studies defined molecular and cellular interaction differences associated with SSc-specific antibodies, including those with anti-RNA polymerase III (ARA) or anti-topoisomerase-1 (ATA).
We examined skin biopsies from well characterized SSc patients to delineate myofibroblast activation by integrating bulk and scRNAsequencing, and quantitative immunostaining for αSMA protein expression.
Methods: 4mm paired skin biopsies were taken from patients with early dcSSc (n=21), and stored for bulk RNAsequencing and histological analysis. Repeat biopsies were obtained at 3 and 12 months. Paraffin embedded biopsies were stained for αSMA and analysed as a proportion of the whole slide. A second cohort of patients (n=12) and healthy controls (HC n=3) donated 4mm skin biopsies, for the purpose of scRNAseq.
Analysis was carried out using R software. Comparisons were made between antibody subsets using ANOVA. Gene set enrichment analysis was performed, with significance at FDR< 0.1.
scRNAseq analysis was carried using packages ‘Seurat’, and ‘gsfisher’.
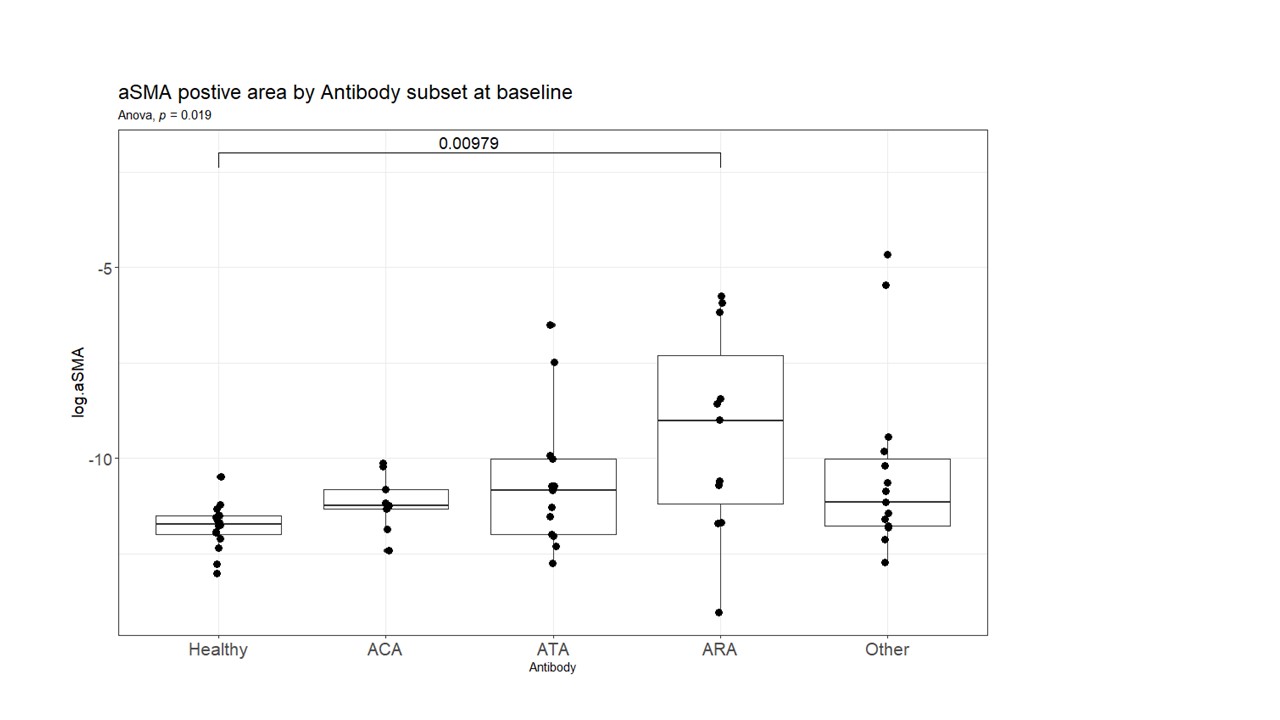
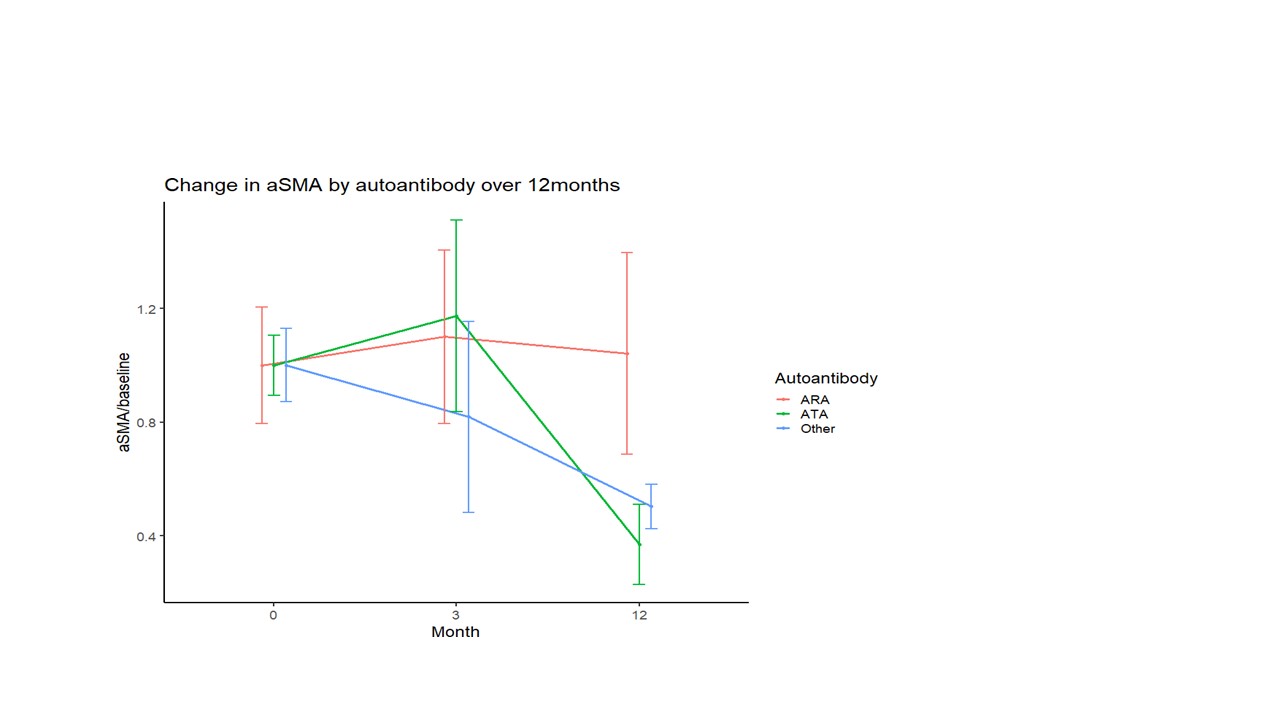
Results: There were significant differences at baseline between αSMA positivity in the skin in ARA+ patients compared to HC (p< 0.01), but no significant difference in the ATA+ subset and HCs (Fig 1). Over time, the proportion of αSMA positive cells declined in the ATA and other antibody subgroups, whereas the ARA+ subgroup of patients showed no difference in αSMA positive cells over 12 months (Fig 2).
Bulk RNA sequencing on whole skin defined significantly enriched pathways shared by ARA+ and ATA+ early dcSSc patients including TGFβ signaling pathway (FDR = < 0.001 and 0.025 respectively), and inflammatory response pathway (FDR = < 0.0001 for both antibody subtypes compared to HC). The PI3K-Akt signaling pathway was only significantly upregulated in ARA+ patients compared to HC (FDR = 0.05), and not in early ATA+ patients (FDR=0.25).
scRNAseq analysis of fibroblast clusters confirmed that this difference in PI3K-Akt signaling pathway enrichment is mainly seen in the early ARA+ patient cohort, and not in the early ATA+ patient cohort, or the late stages of disease.
Conclusion: We have shown significant differences in αSMA positively stained cells in early dcSSc patients compared to HC, more marked in ARA+ than ATA+ patients. Gene expression analysis also confirms key pathways regulating αSMA are upregulated in the ARA+ dcSSc patients and originates from the fibroblast subclusters.
Our results support a stratified approach taking account of ANA subset for targeting myofibroblasts in early stage dcSSc. Our data strongly suggest targeting PI3K-AKT in early-stage ARA+ patients would be beneficial.
To cite this abstract in AMA style:
Clark K, Campochiaro C, Derrett-Smith E, Ong V, Buckley C, Denton C. Integrated Bulk and Single Cell RNA Sequencing Defines Key Pathways Regulating Myofibroblast Differentiation Across ANA Subgroups in Diffuse Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/integrated-bulk-and-single-cell-rna-sequencing-defines-key-pathways-regulating-myofibroblast-differentiation-across-ana-subgroups-in-diffuse-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/integrated-bulk-and-single-cell-rna-sequencing-defines-key-pathways-regulating-myofibroblast-differentiation-across-ana-subgroups-in-diffuse-systemic-sclerosis/