Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Temporary methotrexate (MTX) discontinuation may enhance vaccine immune response, but there are no data for the recombinant zoster vaccine (RZV). This study assessed if a 2-week MTX discontinuation post-vaccine dose could enhance immunogenicity and maintain safety in autoimmune rheumatic disease (ARD) patients.
Methods: This is a single-center, prospective, randomized, investigator-blinded, interventional study, of 202 adult ARD patients under remission/low disease activity for at least 3 months, with stable DMARD dose and prednisone ≤ 5mg/day. Patients were randomized (1:1) to discontinue MTX (MTX-hold) for 2 weeks after each vaccine dose on V1 (day 0) and V2 (day 42) or continue MTX (MTX-maintain). Blood samples were collected before the 1st dose (V1) and 6 weeks after the 2nd dose (V3). The primary endpoint was the enhancement of immune response, and the secondary endpoint was the rate of disease flares at V3. Humoral response was defined as an anti-gE antibody concentration ≥4 times the lower limit of detection (0.02mIU/mL) in initially seronegative subjects, and as an antibody concentration ≥4 times the prevaccination level in initially seropositive subjects ( >0.02 mIU/mL), assessed by an in-house ELISA. Geometric mean titers (GMT) and factor increase in GMT (FI-GMT) were calculated. Flare was defined according to clinical judgment/need for change/increase in therapy or an increase in DAS28-CRP >1.2 or increase in DAS28-CRP >0.6 if baseline DAS28-CRP >3.2 for rheumatoid arthritis (RA); >3 points increase in SLEDAI 2K for systemic lupus erythematosus (SLE); >0.9, increase in ASDAS for axial spondiloarthritis (axSpA) or worsening in DAPSA for psoriatric arthritis (PsA).
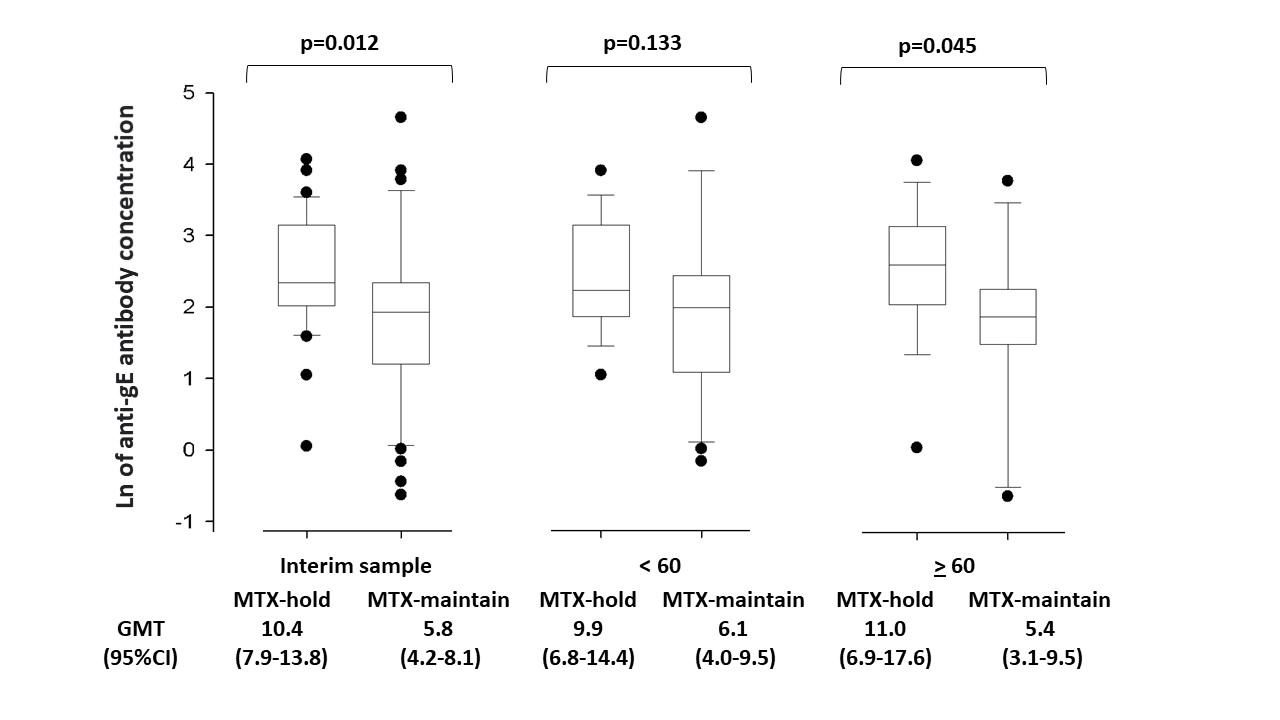
Results: In the interim analysis of 172 ARD patients randomized to date, 75 (32 MTX-hold and 43 MTX-maintain) had complete data for V1/V3: 45 RA, 11 PsA, 10 SLE, 9 other ARDs. Groups were balanced for age (p=0.423), sex (p=0.720), disease distribution (p=0.625), MTX dose (p=0.979), use of glucocorticoid (p=0.615), combined therapy with other immunossupressors (p=0.472), and biological therapies (p=0.270). At V3, MTX-hold and MTX-maintain groups showed similar frequencies of humoral immune responses (96.7% vs. 97.7%; p >0.999). MTX-hold group had higher post-vaccination GMT [10.4 (7.9-13.8) vs. 5.8 (4.2-8.1), p=0.012] and FI-GMT [66.3 (41.1-107.1) vs. 36.3 (24.6-53.5), p=0.049] compared to MTX-maintain. For patients older than 60 years (n=32, 16 in each arm), MTX-hold had higher GMT in comparison to MTX-maintain [11.0 (6.9-17.6) vs. 5.4 (3.1-9.5), p=0.045], but the benefit was not statistical significant for patients under 60 years-old [9.9 (6.8-14.4) vs. 6.1 (4.0-9.5), p=0.133] (Figure 1). Flare rates at V3 (9.4% vs 7.0%, p >0.999) were low and comparable between the groups. No moderate/severe adverse events were reported, with higher frequencies of fever (p=0.033) and chill (p=0.016) after 1stdose in MTX-hold. No cases of herpes zoster were detected up to V3.
Conclusion: A 2-week MTX discontinuation after each dose of the RZV significantly boosts the immune response, without differences in the flare rates. This approach shows promise in enhancing vaccine immunogenicity in ARD patients. (ClinicalTrials NCT05879419)
To cite this abstract in AMA style:
Medeiros-Ribeiro A, de Oliveira J, Aikawa N, Pasoto S, Kupa L, Saad C, Parente L, ASSAD A, Borba E, Figueiredo Neves Yuki E, Shimabuco A, Bonfiglioli K, Domiciano D, Sampaio-Barros P, Franco A, Moraes J, Dorio M, Goldenstein-Schainberg C, Carriço Da Silva H, Guimarães L, Chaer F, Carvalho C, Dalmolin H, Silva C, Bonfa E. Two-Week Methotrexate Discontinuation in Autoimmune Rheumatic Diseases Patients Vaccinated with Recombinant Herpes Zoster Vaccine: An Interim Analysis of a Prospective Randomized Phase 4 Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/two-week-methotrexate-discontinuation-in-autoimmune-rheumatic-diseases-patients-vaccinated-with-recombinant-herpes-zoster-vaccine-an-interim-analysis-of-a-prospective-randomized-phase-4-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/two-week-methotrexate-discontinuation-in-autoimmune-rheumatic-diseases-patients-vaccinated-with-recombinant-herpes-zoster-vaccine-an-interim-analysis-of-a-prospective-randomized-phase-4-study/