Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune checkpoint inhibitors (ICI) have altered the treatment landscape within oncology, with an expanding number of indications. Patients with pre-existing autoimmune disease (PAD) have been largely ineligible for the randomized clinical trials demonstrating ICI efficacy. We previously created a single institution database exploring the use of ICI in patients with PAD to characterize their safety and oncologic outcomes (PMID:37937529). The goal of this analysis was to provide an update on our previous findings with an expanded cohort of patients with PAD.
Methods: We performed a retrospective study of patients treated with ICI therapy between 2012 and 2022 at CancerCare Manitoba (Winnipeg, Canada). Pharmacy records were used to determine ICI exposure. Patient charts were reviewed to determine the existence of a PAD. Demographic, disease, and treatment related variables were captured through retrospective chart review. Patients with PAD disease were matched (1:2) to a control cohort of patients based on age, sex, and cancer type. The primary outcome was immunotoxicity, defined as either a flare of the PAD or a new immune related adverse event (irAE). Other outcomes included objective response rate (ORR), time to treatment failure (TTF) and overall survival (OS) in the subgroup with advanced disease. Time-to-event outcomes were modeled using Kaplan-Meier analysis. Logistic regression was used to examine the association between PAD status and irAEs.
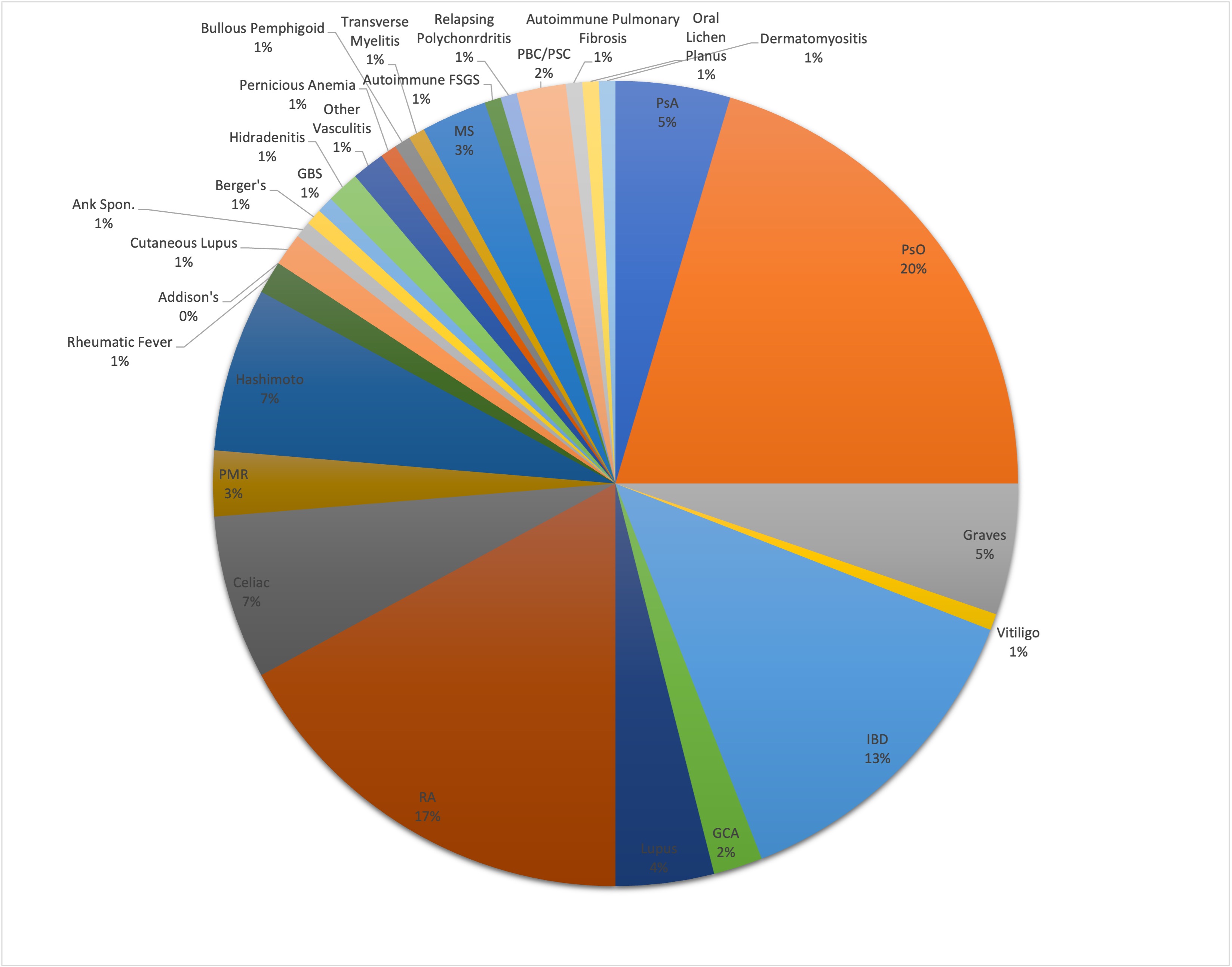
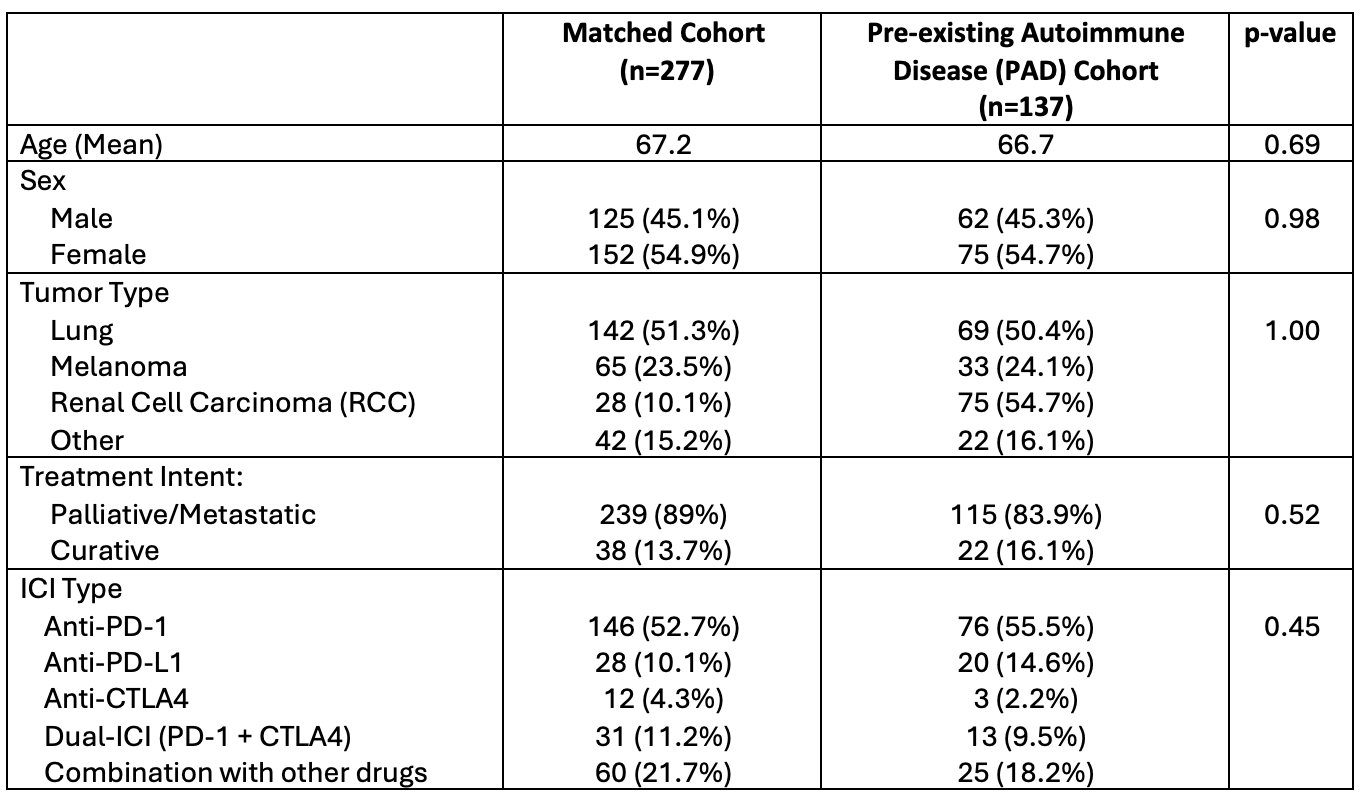
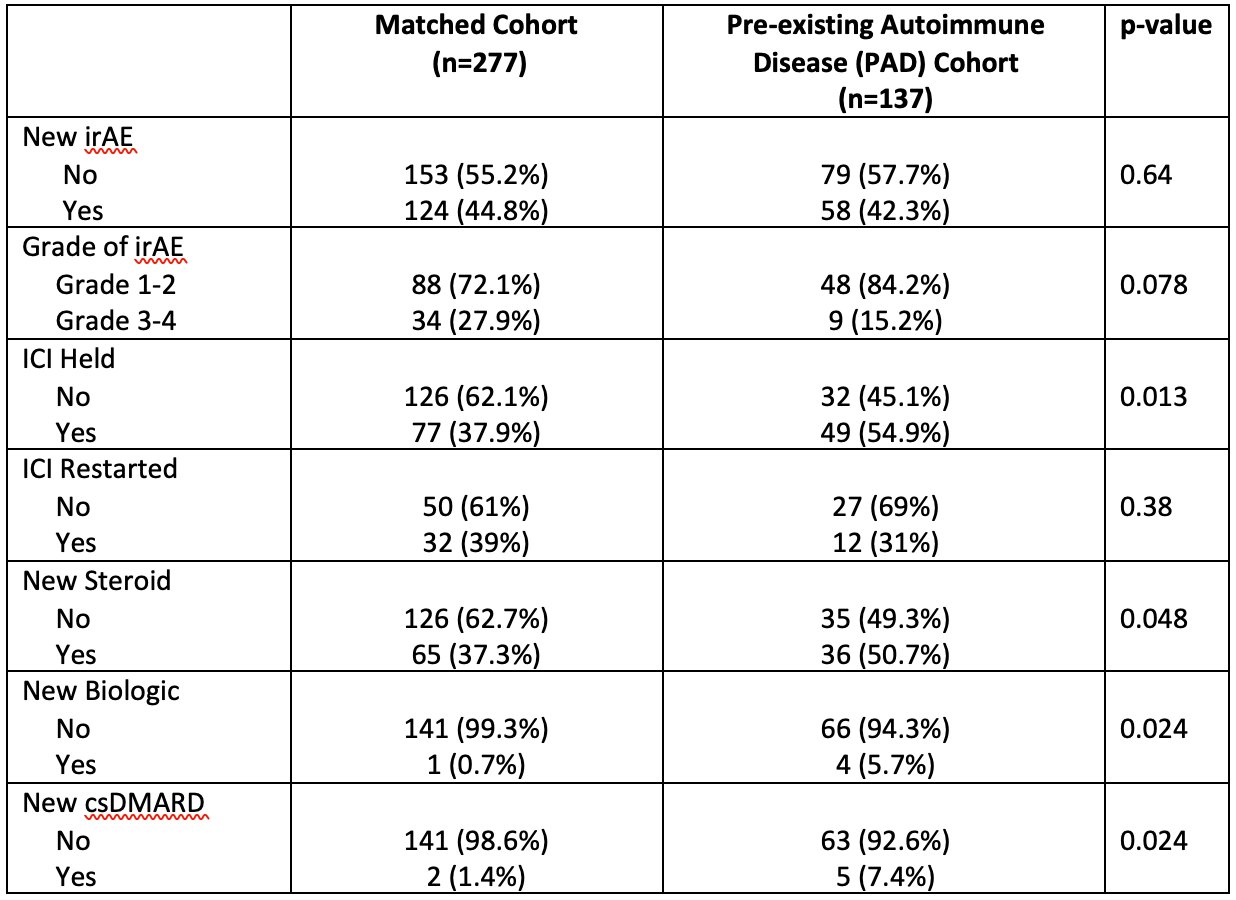
Results: 1891 patients treated with ICI were retrospectively screened for PAD. We identified 137 (7.2%) patients with a PAD, matched to 277 patients without PAD. 26 (19%) of PAD patients had rheumatoid arthritis (RA), 31 (23%) had psoriasis (PsO), 20 (14.5%) had inflammatory bowel disease (IBD) [Figure 1]. At baseline, 23 (17%) patients were on a csDMARD, 9 (6.5%) patients were on systemic steroids, and 2 (2%) patients were on biologic therapy. 55.5% of all patients were treated with PD-1 monotherapy [Table 1]. 71 (51.8%) patients with PAD developed immunotoxicity, including 58 (42.3%) who developed an irAE, 26 (19.0%) who developed a flare of PAD. 13 patients (9.4%) developed both a flare and an irAE. There was no significant association between incidence or grade of irAEs and PAD status (OR: 0.9; 95% CI 0.6-1.4), however a significantly higher proportion of patients with PAD had ICI held in the setting of immunotoxicity (54.9% vs 37.9%, p=0.013). 50.7% of PAD patients were started on steroids compared to 37.3% of non-PAD patients (p=-0.048). Patients with PAD were more frequently started on a csDMARD (7.4% vs 1.4%, p=0.024) or biologic (5.7% vs 0.7%, p=0.024) while on treatment [Table 2]. There was no difference in overall survival, time to treatment failure, or objective response rate between the PAD and non-PAD groups.
Conclusion: Our study demonstrated no significant difference in the incidence or grade of irAEs, in patients with PAD compared to a matched non-PAD group. Approximately 1/5 PAD patients developed a flare while on ICI therapy. PAD patients more commonly required steroid and DMARD administration, however demonstrated similar oncologic outcomes compared to their matched counterparts. Additional analyses will focus on specific PAD subgroups.
To cite this abstract in AMA style:
Goutam S, Raghavan A, Ye C, O'Neil L, Graham J. Safety and Effectiveness of Immune Checkpoint Inhibitor Therapy in Patients with Pre-existing Autoimmune Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-and-effectiveness-of-immune-checkpoint-inhibitor-therapy-in-patients-with-pre-existing-autoimmune-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-effectiveness-of-immune-checkpoint-inhibitor-therapy-in-patients-with-pre-existing-autoimmune-disease/