Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Localized scleroderma is a rare autoimmune disease primarily affecting the skin and underlying tissue. While its exact pathogenesis remains unclear, studies suggest links to systemic sclerosis (SSc) through shared histological features and immune signatures in peripheral blood and skin. The vascular system, particularly endothelial cells, plays a crucial role in SSc pathology, but their role in LS has not been well described. This study aims to characterize endothelial cells in LS and explore their communication with immune cells and contribution to LS pathogenesis.
Methods: Skin biopsy specimens from 27 LS patients and 17 healthy controls were subjected to single-cell RNA sequencing (scRNA-seq). Data were analyzed using the Seurat pipeline. Matrices were processed by CellRanger, converted to Seurat objects for cell annotation, and differential gene expression (DEG) and pathway analyses were performed. Endothelial cell subsets were annotated based on canonical markers, and their intercellular and intracellular interactions were analyzed using the NicheNet R package.
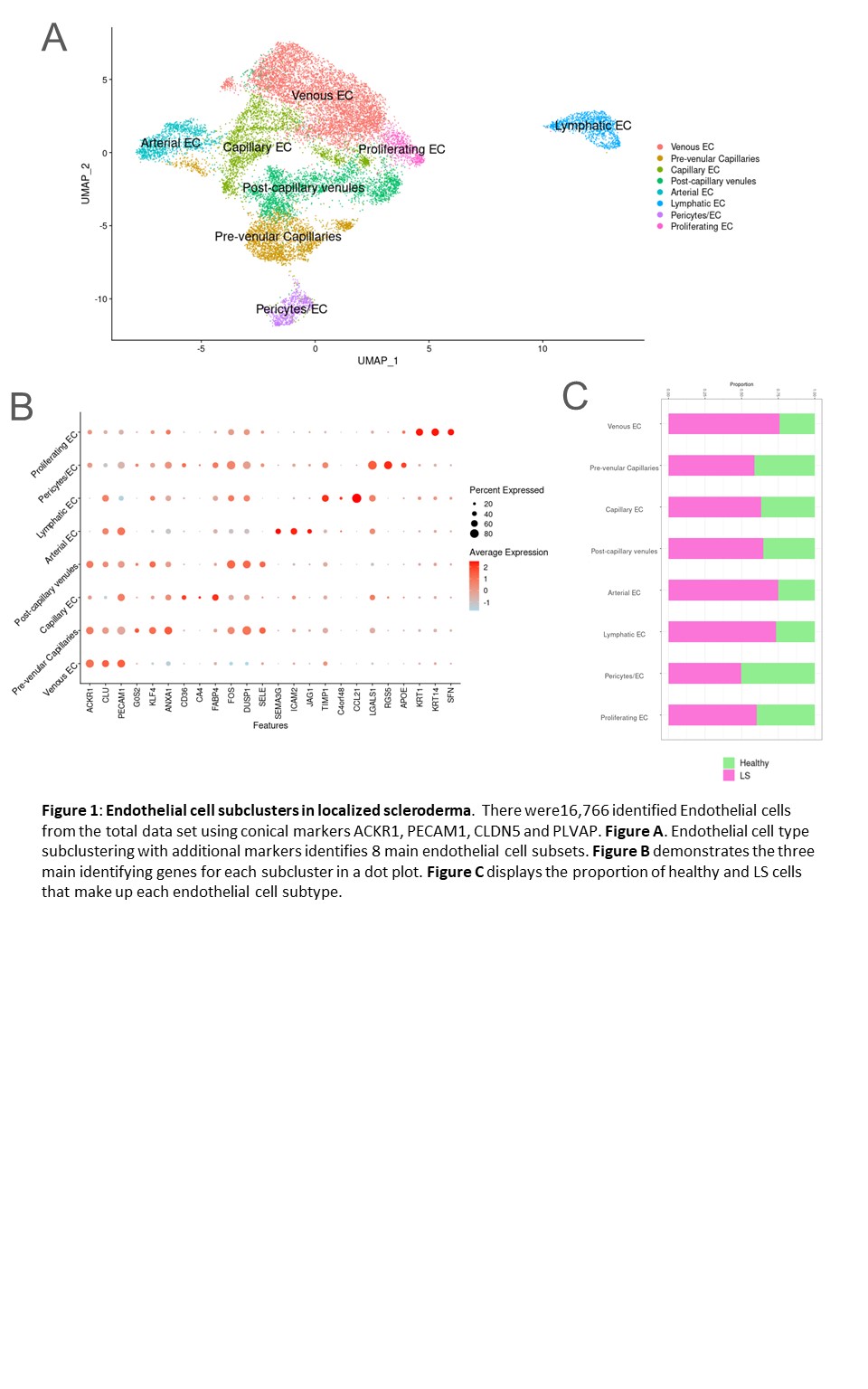
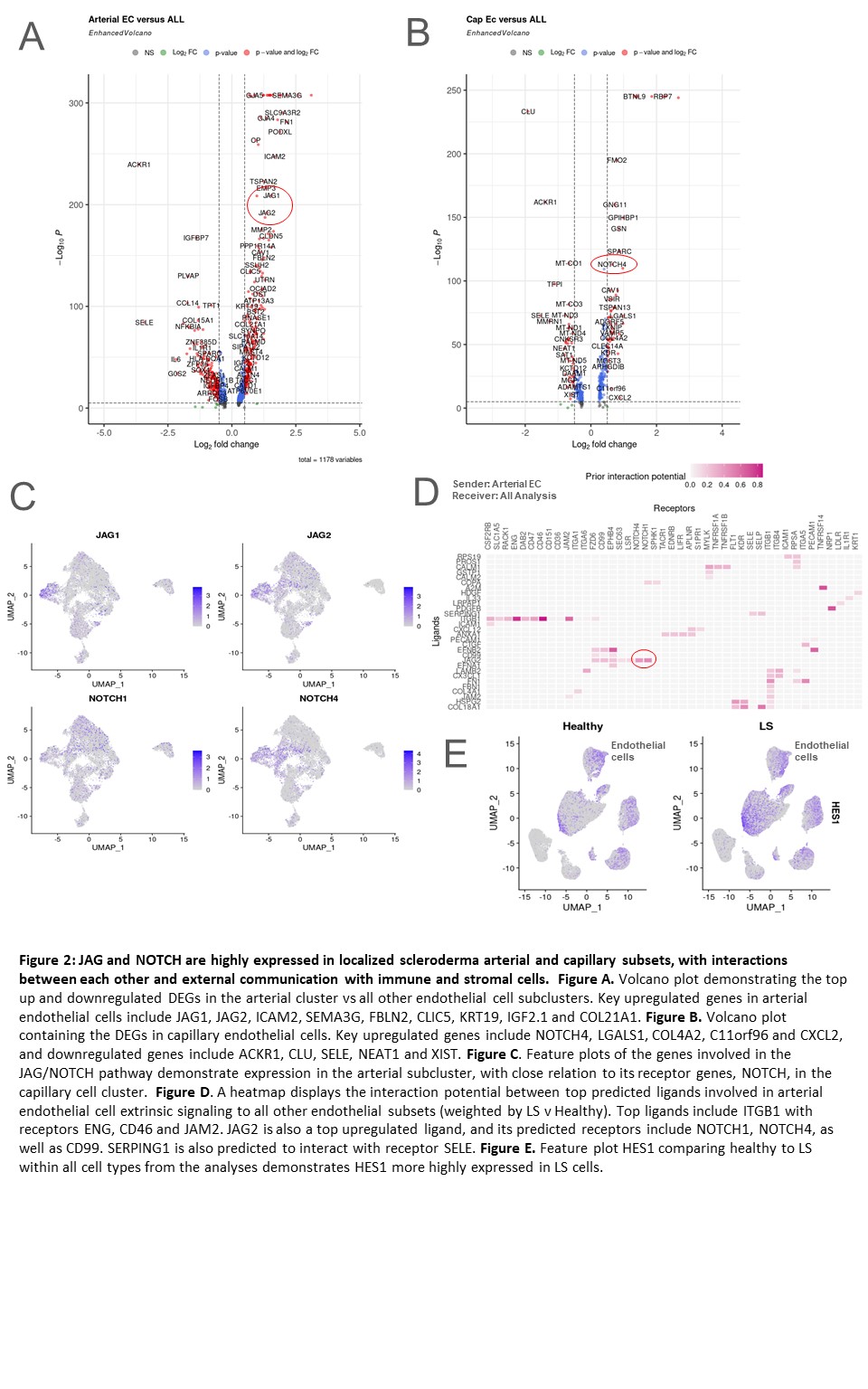
Results: Eight subclusters of endothelial cells were identified in the dataset (LS and healthy skin) using canonical markers (Figure 1A,B). Most populations had a higher proportion in LS compared with HC (Figure 1C). DEG analysis revealed prominent expression of JAG1, JAG2, and NOTCH4 in the arterial and capillary endothelial cell subclusters of LS patients (Figure 2A, B, C). NicheNet analysis predicted significant JAG/NOTCH ligand-receptor interactions within the dataset, supported by a large ligand-target database (Figure 2D). Gene set enrichment analysis (GSEA) indicated that these genes are highly involved in the p53 pathway, which promotes JAG/NOTCH signaling. The upregulation of HES1 in LS cells, a target gene of the JAG/NOTCH pathway, further supports the involvement of endothelial cells in LS pathogenesis (Figure 2E). In addition, JAG1 Is predicted to interact with keratinocytes and smooth muscle cells with signals sent from endothelial cells based on our NicheNet analysis.
Conclusion: Endothelial cells in LS exhibit high expression of the JAG/NOTCH signaling pathway, facilitating communication with other cell types such as smooth muscle cells and keratinocytes. Variants of the NOTCH pathway, previously implicated in pediatric and adult systemic sclerosis, are associated with increased fibrosis and abnormal tissue repair. Similar pathogenic mechanisms may be at play in LS, highlighting the potential role of JAG/NOTCH signaling in its pathogenesis.
To cite this abstract in AMA style:
Hutchins T, Sanyal A, Esencan D, Torok K. Endothelial Cell-Driven JAG/NOTCH Signaling in Localized Scleroderma Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/endothelial-cell-driven-jag-notch-signaling-in-localized-scleroderma-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/endothelial-cell-driven-jag-notch-signaling-in-localized-scleroderma-patients/