Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Despite considerable advances in pharmacologic approaches to RA management, a better understanding of the underlying immunopathology is still needed.1,2 A dysregulated immune system is the basic feature of RA, reflected by the complex interplay of elevated autoreactive CD4+ T cells, pathogenic B cells, and macrophages with inflammatory cytokines, chemokines, and autoantibodies.3 Abatacept (ABA) and adalimumab (ADA), both effective therapies, have distinct mechanisms of action, and likely have different effects on immune homeostasis that could inform selection of suitable treatment (tx) options for patients with RA. We investigated the differential impact of these 2 agents on relevant immune cells in patients with ACPA+ early RA (NCT04909801).
Methods: This phase 3, randomized, single-blind study evaluated the clinical efficacy of ABA vs ADA in patients with early RA who had inadequate response to MTX, dual seropositivity for ACPA and RF, and shared epitope HLA class II risk alleles.4 Flow cytometry analysis was conducted on samples collected from patients at different timepoints (baseline, week 12, and week 24) to investigate immune subsets in peripheral blood, including T cells, B cells, NK cells, and monocytes. Raw unadjusted means and associated 95% CIs were plotted. Wilcoxon rank-sum test was used to test statistical significance and generate P values.
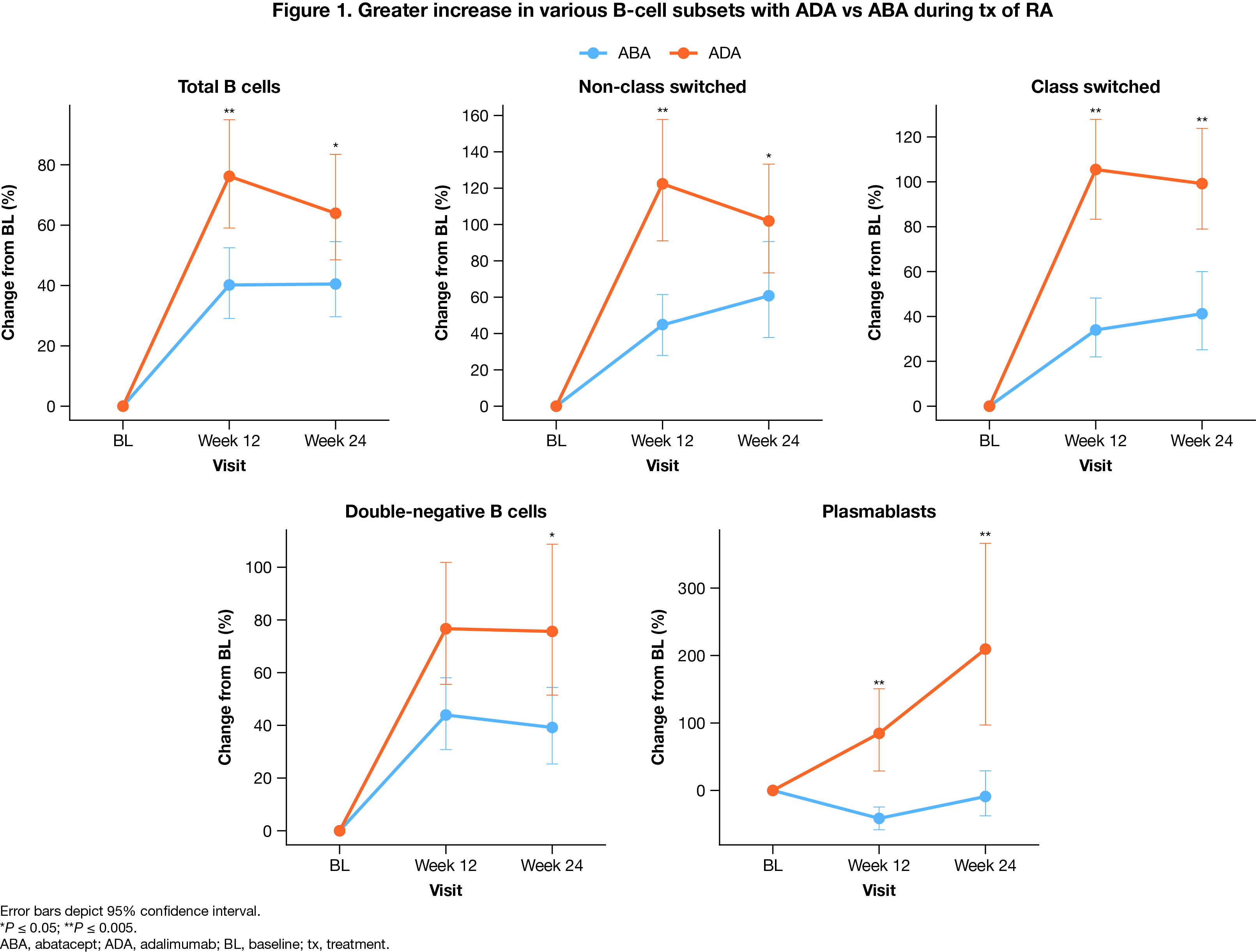
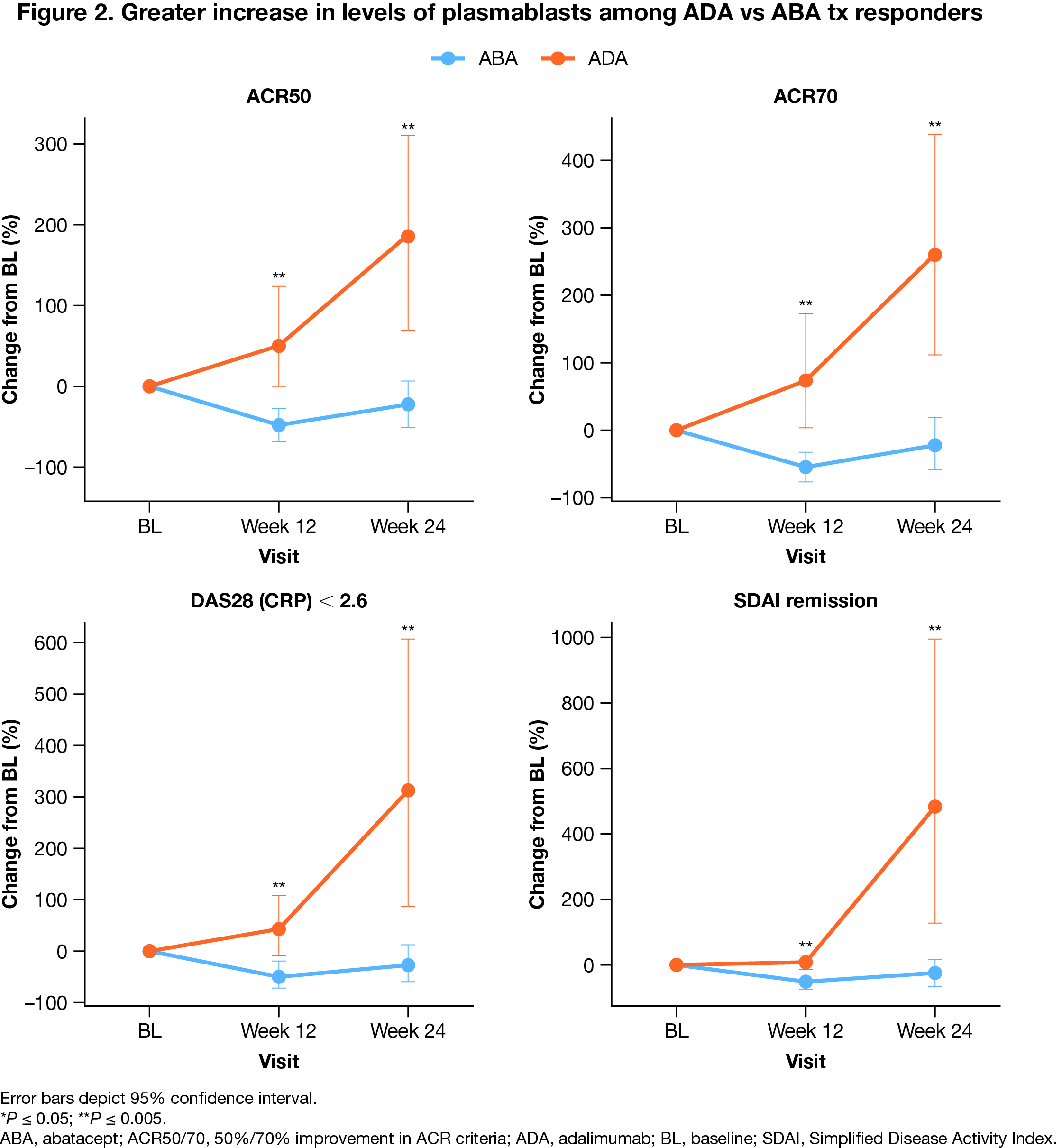
Results: A total of 338 patients were randomized (n = 169 per arm). Baseline characteristics were balanced between tx arms. Efficacy at 24 weeks was comparable in both tx arms. Decreases in CCP2 and RF titers were more notable in the ABA arm. From baseline to weeks 12 and 24, ABA tx resulted in a greater increase in naive CD4+ and CD8+ cells, naive B cells, and Th2 cells, while ADA tx increased Th1, Th17, and Th17.1 cells. Compared with ABA, ADA tx led to greater increase in B-cell subsets, including total B cells, non‑class switched and class-switched memory B cells, double-negative B cells, and plasmablasts (Fig 1). Among tx responders, defined by ACR50, ACR70, DAS28 (CRP) < 2.6, and Simplified Disease Activity Index remission, an increase in class-switched memory B cells and plasmablasts was seen in patients who received ADA vs ABA tx (Fig 2). In comparison to ABA, an increase in Th1 and Th17 cells was seen among ADA responders. Differential impact on other immune subsets is currently under investigation.
Conclusion: This analysis of immune cell subsets in patients with ACPA+ early RA reveals different patterns in immune system modulation between ABA and ADA txs, despite overall similar efficacy outcomes. While ABA tx promoted increased levels of naive T and B cells, ADA responders demonstrated higher levels of Th1, Th17, and B cells, including switched memory B cells and plasmablasts. This is the first study to investigate the modulating effects of ABA vs ADA tx on B cells. Further investigation is warranted to understand the clinical significance of these findings.
References:
1. Radu AF, et al. Cells 2021;10:2857.
2. Di Matteo A, et al. Lancet 2023;402:2019–2033.
3. Jang S, et al. Int J Mol Sci 2022;23:905.
4. Emery P, et al. Presented at EULAR 2024; June 12−15, 2024. Oral OP0007.
Medical writing: Sameen Yousaf, PhD (Caudex), funded by Bristol Myers Squibb.
To cite this abstract in AMA style:
Eddy N, Buckner J, Emery P, Weinblatt M, Bykerk V, Cope A, Burmester G, Tanaka Y, Citera G, Nash P, Dornic Q, Schafer P, Kelly S, Maldonado M, Liu J. Differential Pharmacodynamic Changes of Circulated Immune Subsets After Treatment with Abatacept or Adalimumab in Patients with ACPA+ Early RA in the AMPLIFIED Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/differential-pharmacodynamic-changes-of-circulated-immune-subsets-after-treatment-with-abatacept-or-adalimumab-in-patients-with-acpa-early-ra-in-the-amplified-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/differential-pharmacodynamic-changes-of-circulated-immune-subsets-after-treatment-with-abatacept-or-adalimumab-in-patients-with-acpa-early-ra-in-the-amplified-study/