Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Interstitial lung disease (ILD) is a common complication of connective tissue disease (CTD) with a high prevalence in SSc and inflammatory myopathy (IM). Early identification and intervention is critical to reduce the significant morbidity and mortality associated with CTD-ILD. Computed tomography is currently the gold standard for screening and diagnosis but has the drawback of high cost, significant radiation exposure, imaging delays, negative environmental impact, and may not be readily available in resource limited areas. Lung ultrasonography has shown promise as an alternative screening modality that circumvents these drawbacks. We previously developed lung ultrasound interpretation criteria for ILD screening (LUS-ILD-20) showing excellent sensitivity and specificity in a pilot study in SSc-ILD; in this study we sought to validate a revised LUS-ILD-24 in a large SSc and IM cohort.
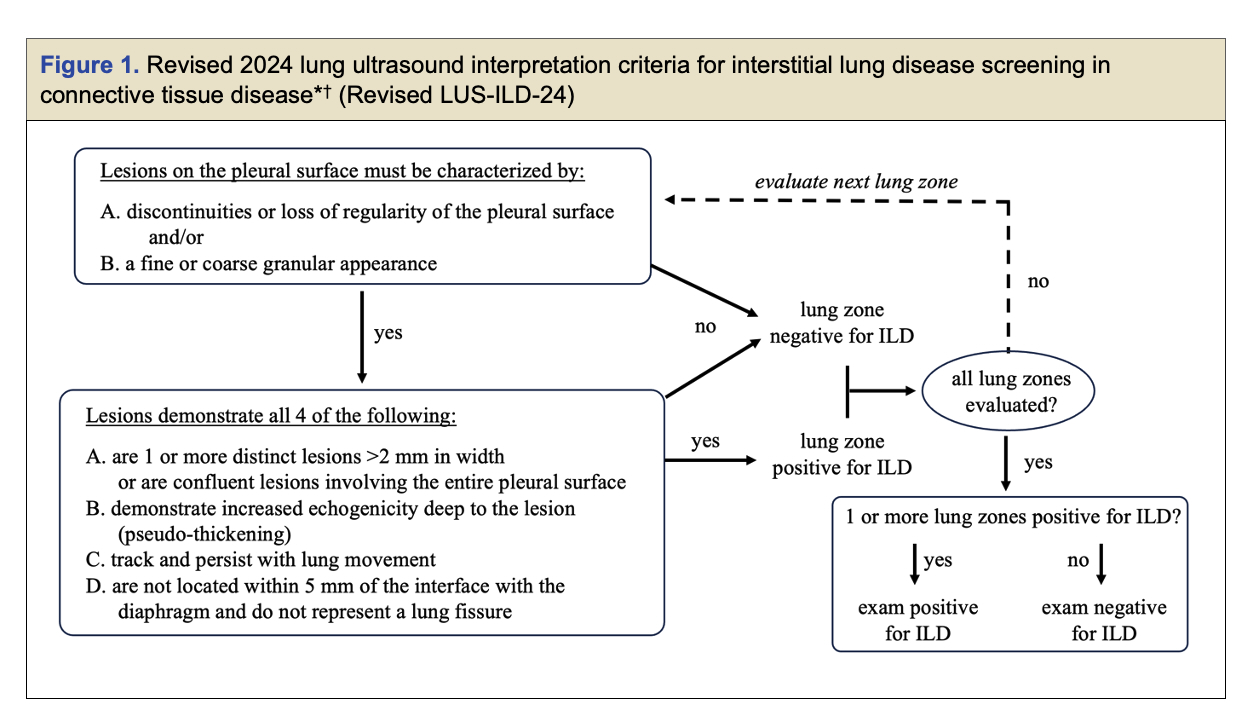
Methods: Our prior pilot LUS-ILD-20 criteria was minimally revised to improve clarity and account for physiologic pleural findings. To validate the revised LUS-ILD-24 criteria (Figure 1), patients meeting ACR criteria for SSc and IM, with planned CT chest imaging and pulmonary function tests (PFTs) were enrolled. All patients underwent LUS imaging (14 lung zones, 4 second cine clips) within 10 days of CT and 3 blinded readers independently interpreted the LUS imaging using the revised LUS-ILD-24. CT imaging was read by a radiologist for the presence or absence of ILD and findings on CT were quantified using CALIPER lung texture analysis (Computer-Aided Lung Informatics for Pathology Evaluation and Rating). The sensitivity and specificity for CTD-ILD detection as noted on CT was analyzed for individual readers and consensus reading for all 3 readers. Additional analyses were performed on subgroups of SSc, IM, and “possible-ILD” (patients with no prior ILD). Inter- and intra-rater agreement was assessed with Randolph’s free marginal kappa and Cohen’s kappa. Spearman correlations between LUS severity scoring (sum of positive views) and both PFT indices and CALIPER quantification were analyzed.
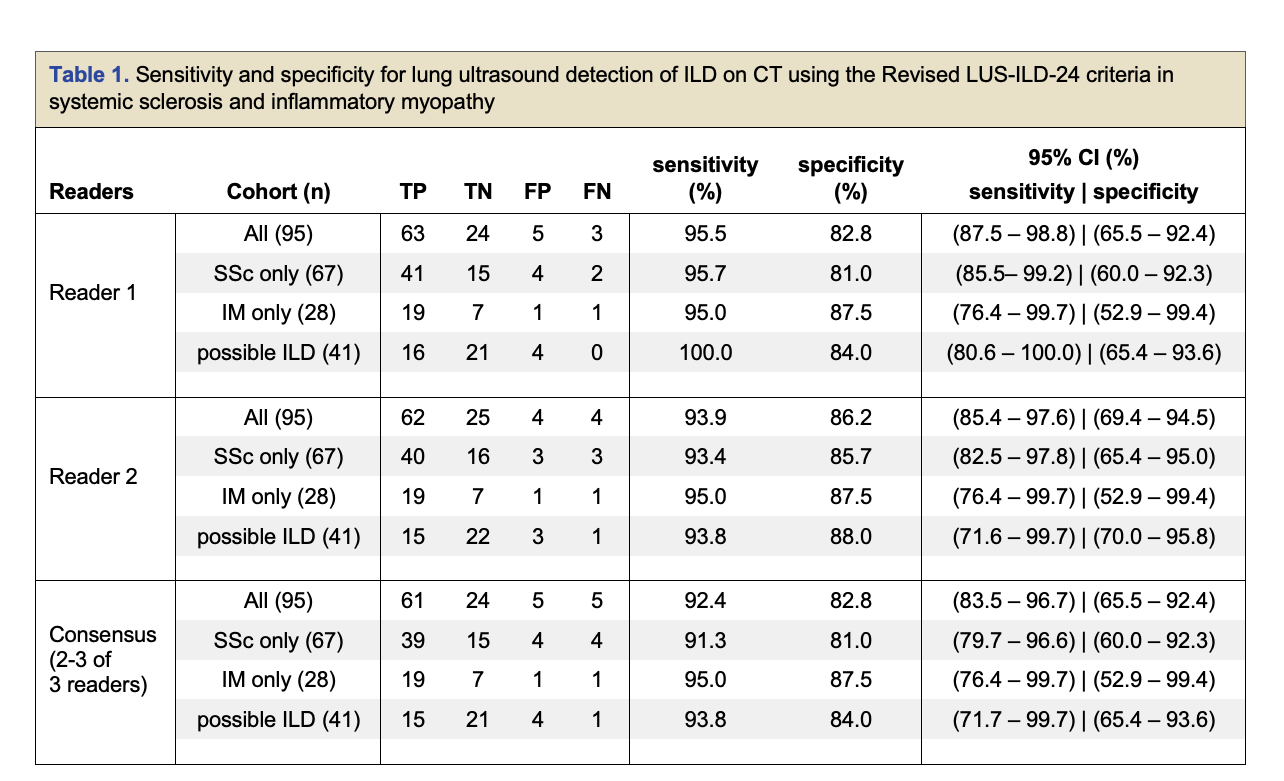
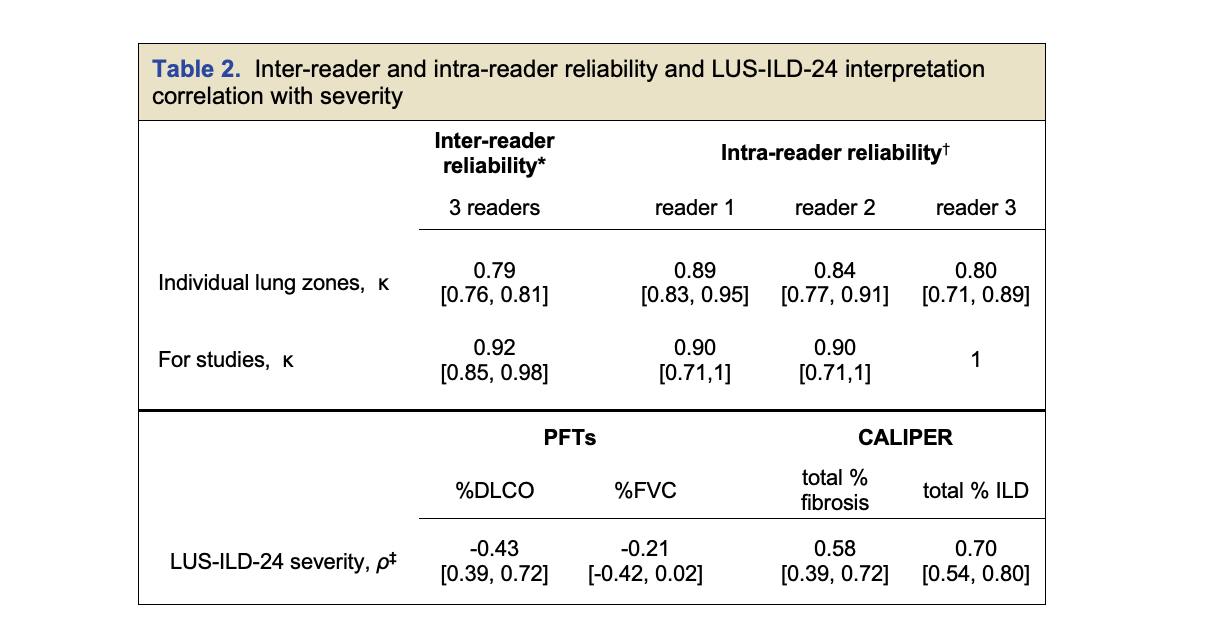
Results: We enrolled 100 patients, 72 with SSc and 28 with IM, and analyzed 95 after excluding 5 patients for active non-ILD pathology on CT scan. All excluded patients were LUS(+) across readers. Sensitivity and specificity ranged from 92.4% to 95.5% and 82.8% to 86.2% for individual and consensus reading for the 95 patient cohort (Table 1). Subgroups, including the “possible-ILD” group showed similar accuracy. Intra-reader reliability showed near perfect agreement across 3 readers for study interpretation ( κ = 0.92 with 95.8%). Intra-reader agreement for studies for each reader was similarly high (κ = 0.90 to 1) (Table 2). LUS severity correlated with CALIPER “percent fibrosis” and “percent ILD” indices and inversely correlated with %DLCO but not FVC.
Conclusion: We validated our revised LUS-ILD-24 in SSc and IM cohorts and found excellent sensitivity, specificity, and reliability for detection of ILD identified on CT. LUS severity correlated with CT and PFT markers of ILD severity. Validation of the revised LUS-ILD-24 opens the door to implementation of LUS as a potential replacement for CT for ILD screening in this population.
To cite this abstract in AMA style:
Fairchild R, Mar D, Deluna M, Guo H, Fiorentino D, Chung L. Validation of a Lung Ultrasound Interpretation Criteria for Interstitial Lung Disease Screening in Systemic Sclerosis and Inflammatory Myopathy [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/validation-of-a-lung-ultrasound-interpretation-criteria-for-interstitial-lung-disease-screening-in-systemic-sclerosis-and-inflammatory-myopathy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-a-lung-ultrasound-interpretation-criteria-for-interstitial-lung-disease-screening-in-systemic-sclerosis-and-inflammatory-myopathy/