Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Janus kinase inhibitors (JAKIs) have shown positive therapeutic impacts on treatments for rheumatoid arthritis (RA), whereas, there are concerns about the risk of malignancy based on the results from the clinical trials with a limited number of patients. Thus, a population-based study in clinical settings is needed to investigate the safety of JAKIs in the real-world. The purpose of this study was to compare the risk of malignancy among JAKIs users, biological disease-modifying antirheumatic drugs (bDMARDs) (tumor necrosis factor inhibitors [TNFIs] and non-TNFIs) users, and methotrexate (MTX) users in patients with rheumatoid arthritis (RA) using a large health insurance claims data in Japan.
Methods: This retrospective longitudinal population-based study was conducted using claims data provided by Medical Data Vision Co., Ltd (Tokyo, Japan). We defined individuals as new users of JAKIs (tofacitinib, baricitinib, peficitinib, upadacitinib, and filgotinib), bDMARDs or MTX if they met all of the following: 1) having at least one ICD10 code, M05 or M06; 2) having at least one prescription of JAKI, bDMARDs, or MTX between July 2013 and July 2020; 3) being over 18 years old; and 4) having prescription of neither JAKIs, bDMARDs nor MTX prior to six months before the index month (i.e., baseline period). The index month was defined as the first month in which cases met all of the above criteria of 1), 2), and 3). Patients were followed from the index month until the last exposure to JAKIs, bDMARDs or MTX, date of loss of follow-up, or the end of follow-up (July 2021), whichever came first. Occurrence of malignancy was defined when patients had at least one ICD code of malignancy and chemotherapy, radiation, or surgery for malignancy. Patients were excluded from the study population if they had a history of malignancy during the baseline period. We defined baseline characteristics using the data during the baseline period, and calculated incidence rate (IR), IR ratio (IRR, JAKIs vs. TNFIs, JAKIs vs. non-TNFI, JAKIs vs. MTX), and adjusted hazard ratio (HR [95% CI]) of the malignancy using a multivariable Cox regression model after adjusting for age at index month, sex, and comorbidities at baseline.
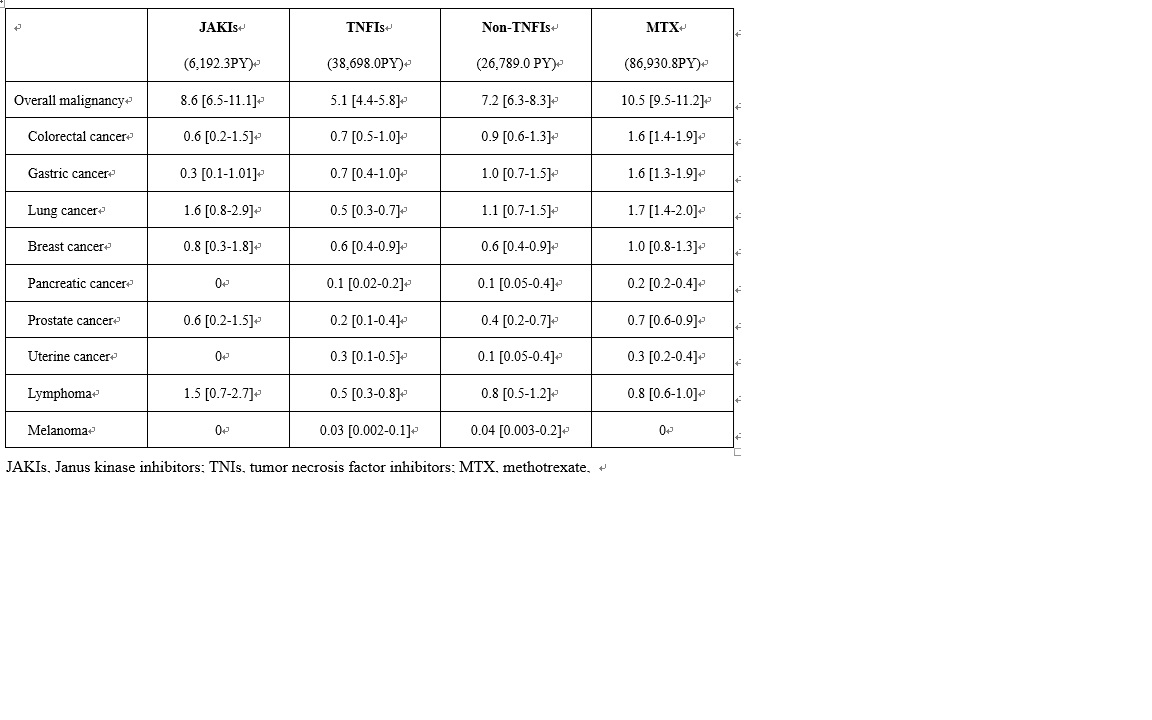
Results: In this study, 52,582 cases were included. The mean age was 64 years and 73% were female. Median observation period was 32 months. IRs/1,000 patient-years (PY) of the overall malignancy are shown in Table 1. Crude IRR of overall malignancy of JAKIs vs. TNFIs was significantly increased (1.7 [1.2-2.3]), whereas IRRs of JAKIs vs. non-TNFIs (1.2 [0.9-1.6]) as well as JAKIs vs. MTX (0.8 [0.6-1.1]) were not. Adjusted HR of JAKIs for the overall malignancy was 2.2 [1.6–3.1] versus TNFIs, 1.6 [1.2–2.2] versus. non-TNFIs, and 1.2 [0.9–1.6] versus MTX.
Conclusion: JAKIs user had a significantly higher risk of the overall malignancy than TNFIs and non-TNFIs users, and a similar risk to MTX users in patients with RA.
To cite this abstract in AMA style:
Sakai R, Tanaka E, Inoue E, Harigai M. Risk of Malignancy Under the Treatments with Janus Kinase Inhibitors in Patients with Rheumatoid Arthritis: An Analysis Using Japanese Health Insurance Database [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/risk-of-malignancy-under-the-treatments-with-janus-kinase-inhibitors-in-patients-with-rheumatoid-arthritis-an-analysis-using-japanese-health-insurance-database/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-malignancy-under-the-treatments-with-janus-kinase-inhibitors-in-patients-with-rheumatoid-arthritis-an-analysis-using-japanese-health-insurance-database/