Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Interleukin-6 (IL-6) is a major inflammatory cytokine involved in pain and potentially in osteoarthritis (OA) disease progression. PPV-06 is an IL-6-derived peptide coupled with a carrier protein, CRM197. The goal of the PPV-06 active immunotherapy is to induce the production of anti-IL-6 antibodies to buffer its biological activity. The PPV-06 conjugate has been shown to be safe in preclinical studies. We report here the results of a phase 1 clinical trial (First-in-human).
Methods: This study (NCT04447898) was a randomized, double-blind, placebo (PBO)-controlled in adults (≥40 y) with Knee OA per ACR criteria, moderate-to-severe pain and signs of knee effusion, Kellgren and Lawrence (KL) score≥ 2, BMI of 18-32 kg/m2. Patients were randomized (3:1) in: a) Cohort 1: Low dose (10 μg per SC injection) = 9 PPV-06 and 3 PBO; or b) Cohort 2: High dose (50 μg per SC injection) = 9 PPV-06 and 3 PBO. Each patient received 3 injections of PPV-06/PBO (at Day 1, at W4 and W16) and were followed during 6 months after the last injection. The primary endpoint was the proportion of patients with Dose-Limiting Toxicities (DLTs) defined as Grade ≥3 Treatment-Emergent Adverse Events (TEAEs) of special interest (anaphylactic shock, allergy, and occurrence of infection) during the study. The secondary endpoints were the occurrence of AEs and SAEs, quantification of inflammatory markers (IL-6, hsCRP, CRPM), quantitative analysis of the B-cell immune response and existence of a cellular immune response. Exploratory endpoints were the quantification of OA markers (C1M, C2M, C3M, ARGS, PRO-C2, CTX-II), changes in the Knee Injury and OA Outcome Score (KOOS) and pain scale on Numeric Rating Scale (NRS) assessed at baseline and EoS.
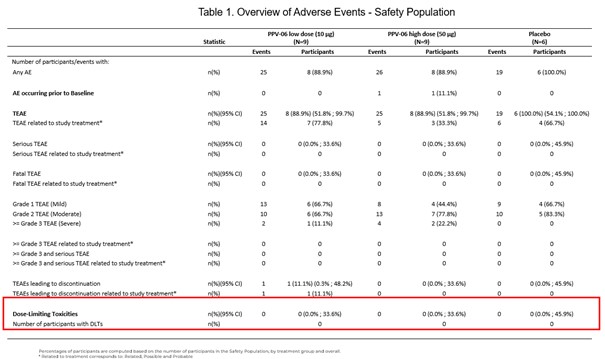
Results: Overall, 24 patients were randomized and no patient experienced a DLT during the study. AEs induced by PPV-06 administration were mostly transient and of mild to moderate severity (injection-site pain was commonly reported). All AEs were TEAEs and one patient reported an AE leading to study discontinuation (Grade 2 induration at injection site). Neither serious TEAEs nor SAEs were reported (Table 1). Anti-IL-6 neutralizing antibodies were detected in both treated groups, but not in the PBO group. Transient increases of IL-6 levels compared to hsCRP were observed in the PPV-06 treated groups, reflecting the presence of immune complexes and their capacity to reduce inflammation. No T-cell responses were observed against the IL-6 peptide of the PPV-06 conjugate, nor against the full human IL-6 protein, indicating the absence of autoreactive T cells post-vaccination. Serum biomarkers for OA showed a downward trend in C2M and C3M levels, in the PPV-06 groups compared to PBO. A trend towards improvement of all KOOS scores was observed in the PPV-06 groups compared to PBO, more prominently for QoL and Symptoms. NRS pain score remained unchanged throughout the study.
Conclusion: The PPV-06 active immunotherapy administered to patients with inflammatory KOA at two dose levels (10 µg and 50 µg) demonstrated a favorable safety profile and the evidence of immunogenicity in both PPV-06 treated groups. This outcome represents a significant milestone in the development of the PPV-06 active immunotherapy program.
To cite this abstract in AMA style:
Rannou F, Nguyen C, Daste C, Kirren Q, Lefevre-Colau m, Launay O, Desallais L, Dolimier E, Azoulai R, Salles J, Zagury J. Safety and Immunogenicity of an Active Anti-IL-6 Immunotherapy in a Phase 1 Clinical Trial in Knee Osteoarthritis Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-and-immunogenicity-of-an-active-anti-il-6-immunotherapy-in-a-phase-1-clinical-trial-in-knee-osteoarthritis-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-immunogenicity-of-an-active-anti-il-6-immunotherapy-in-a-phase-1-clinical-trial-in-knee-osteoarthritis-patients/