Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis associated interstitial lung disease (RA-ILD) is the most overrepresented cause of death among RA patients, yet the mechanisms underlying lung fibrosis remain elusive. Recent studies have highlighted the role of malondialdehyde-acetaldehyde (MAA)-modified proteins in the pathogenesis of RA-ILD. This modification acts as a potent hapten, triggering autoimmune responses that are predictive of RA-ILD. Moreover, MAA-adducted proteins are enriched in lung tissues of RA patients and together with citrulline (CIT) induce macrophage-fibroblast cross talk leading to increased extracellular matrix deposition. The aim of this study was to examine whether immunization of mice with MAA-modified protein induces lung fibrosis.
Methods: Arthritis prone male DBA/1J mice (n=5/group) were given weekly intraperitoneal injections for 5 weeks consisting of: 1) 25μg/mL of unmodified vimentin (VIM), 2) 25μg/mL of VIM-MAA, or 3) equal volumes of saline (additional negative control). Mice were sacrificed the sixth week. Lung tissues were resected, paraffin embedded, sectioned, and stained with trichrome (for total collagen deposition) or immunohistochemistry (IHC) using anti-MAA, anti-CIT, and anti-VIM antibodies. Dissociated lung tissues underwent analysis by flow cytometry to characterize immune cell infiltration. Mice were assessed for arthritis development using a semi-quantitative score that incorporates paw swelling and redness. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparisons test.
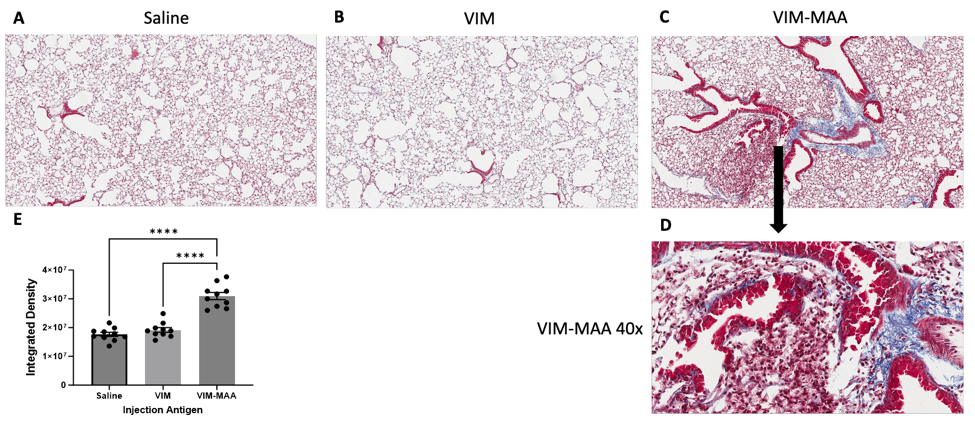
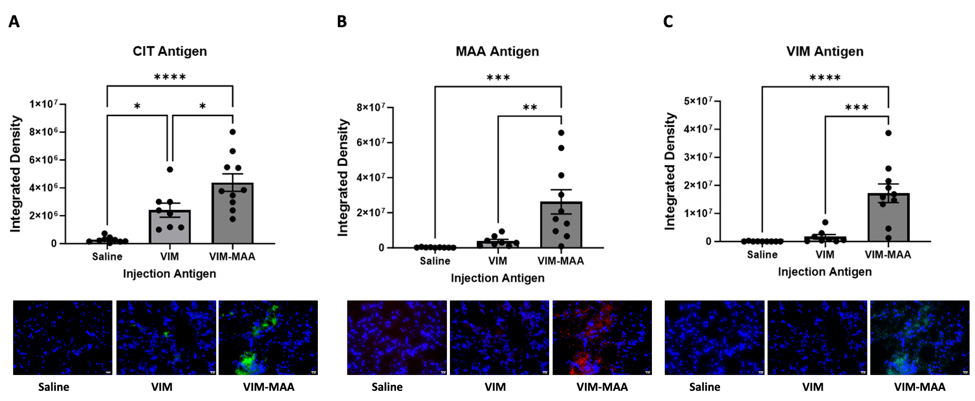
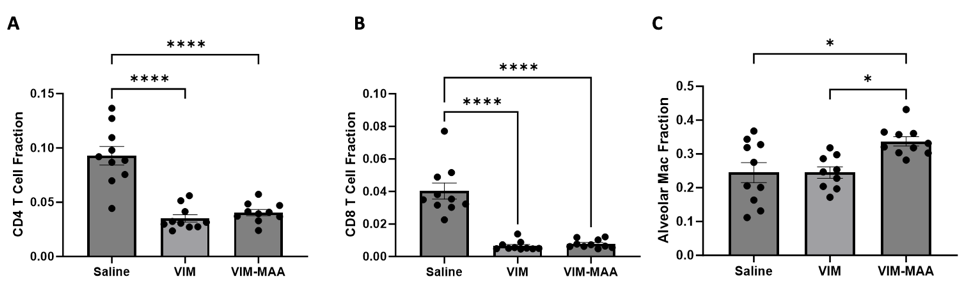
Results: Trichrome staining of lungs revealed increased collagen deposition in VIM-MAA immunized mice (vs. VIM or saline, p< 0.0001) that was most prominent around sites of cellular infiltrate (Fig.1). Likewise, IHC staining demonstrated the highest expression of CIT (P< 0.05), MAA (p< 0.01), and VIM (p< 0.001) antigens in the lungs of VIM-MAA immunized mice (Fig.2). MAA-modified proteins were co-localized with both CIT (r2 = 0.46) and VIM (r2 = 0.33). Flow cytometry of lung tissue revealed a significant increase in alveolar macrophages with VIM-MAA immunization vs. other groups (Fig. 3, p< 0.05) but no other changes in myeloid cell subpopulations. In contrast, there was a decrease in CD4/CD8 T cells in mice immunized with VIM or VIM-MAA compared to saline controls (p< 0.0001). There were no differences in arthritis scores across groups.
Conclusion: This study is the first to demonstrate that systemic immunity to MAA-modified vimentin directly contributes to lung fibrosis. In addition to demonstrating increased collagen and vimentin deposition, affected tissues were characterized by increased alveolar macrophages and expression of both MAA- and CIT-modified proteins accompanied by decreased T cell populations. An improved understanding of mechanisms linking VIM-MAA immunization with lung fibrosis using this model may provide much needed insight into the pathogenesis of RA-ILD.
To cite this abstract in AMA style:
Zhou W, Duryee M, Aripova N, Poole J, Hunter C, Nelson A, England B, Mikuls T, Thiele G. Immunization of Arthritis Prone Mice with Malondialdehyde-Acetaldehyde (MAA) Modified Vimentin Induces Fibrotic Lung Changes [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/immunization-of-arthritis-prone-mice-with-malondialdehyde-acetaldehyde-maa-modified-vimentin-induces-fibrotic-lung-changes/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunization-of-arthritis-prone-mice-with-malondialdehyde-acetaldehyde-maa-modified-vimentin-induces-fibrotic-lung-changes/