Session Information
Date: Sunday, November 17, 2024
Title: Innate Immunity Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: NLRP3 is emerging as an attractive upstream target of the pathway to down-modulate rather than to completely neutralize IL-1ß levels in both acute and chronic inflammatory conditions. Such goldilocks properties are made possible by discovery of specific NLRP3 inhibitors that confer a greater degree of specificity compared to injectable IL-1 directed monoclonal antibodies, together with excellent oral bioavailability and little or no off-target liability. As the therapeutic possibilities for application of specific NLRP3 inhibitors such as dapansutrile (OLT1177) in multiple acute and chronic inflammatory diseases have increased, so has the need to develop objectives tests to screen possible drug candidates in this class.
Recently, the NLRP3 NanoBRET® target engagement assay has been described as a method to measure direct NLRP3 engagement1. The authors tested NLRP3 inhibitors in this new assay and concluded that of the seven NLRP3 inhibitor tested, one (Cy09) showed only partial engagement, and two (OXSI-2 and OLT1177) failed to show direct antagonism. Of these three, we are particularly familiar with OLT1177 (dapansutrile). Our group and other independent investigations have demonstrated that OLT1177 is a specific NLRP3 inhibitor2. OLT1177 is currently in two large phase 2 trials of 300 subjects each in acute gout flare PODAGRA-II (NCT05658575) and in type 2 diabetes with low-grade inflammation DAPAN-DIA (NCT06047262) in a consortium of centers of excellence for understanding the role of IL-1 mediated inflammation in cardio-metabolic disease. An additional trial in early Parkinson’s Disease at the Univ of Cambridge is planned to start soon.
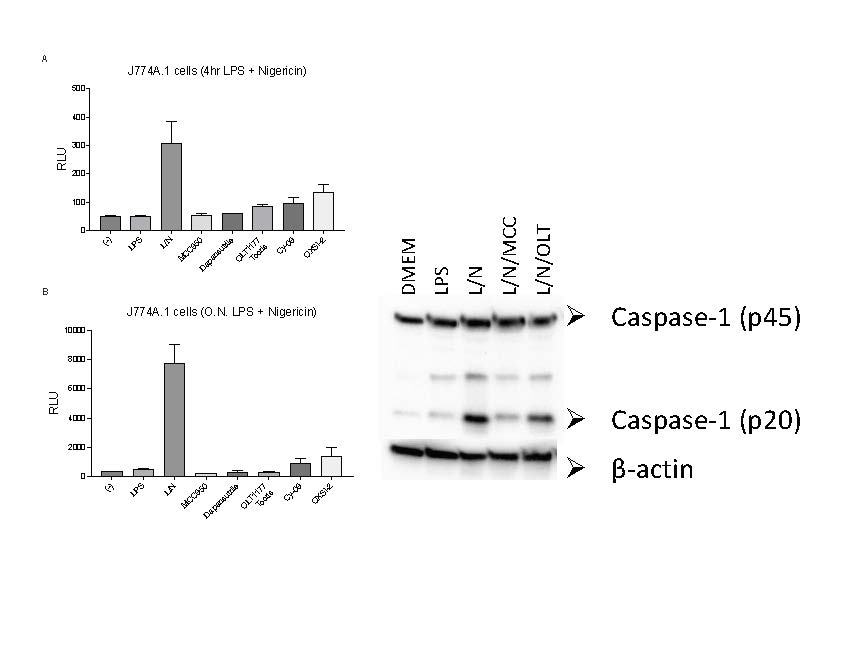
Methods: We measured IL-1β levels using the same detection assay used by Teske et al under canonical NLRP3 stimulation (LPS 4 hours at 1µg/ml and nigericin 10µM for 60 minutes) as well as priming the cells overnight (LPS 500ng/ml) as reported.
Results: Each of the NLRP3 inhibitors were tested at 10µM. Consistent with the Teske et al, and our previous data, MCC950, a sulfonylurea-based compound showed the strongest inhibition (Figure 1). Contrary to the data reported by Teske et al, both OXSI-2 and dapansutrile decreased IL-1β secretion as detected by the Lumit Mouse IL-1β Immunoassay (Figure 1A, B). Western blots exhibited a reduction in the levels of active caspase 1 (p20) by OLT1177® when compared to cells treated with LPS and nigericin (Figure 1C). OLT1177®, a non-sulfonylurea compound, reduced NLRP3’s ATPase activity and directly binds NLRP3 with a KD of 1.2µM in cell free assay2. However, relative to MCC950, dapansutrile has a 5-fold lower affinity binding (0.224µM vs 1.2µM respectively).
Conclusion: The most clinically relevant promise of NLRP3 inhibitors as an emerging new therapeutic class is to safely control inflammation when administered chronically. Although new screening technologies such as NanoBRET® have been proposed, they come with relevant caveats and assay-specific limitation that must be understood for interpretation, clinical relevance and generalizability to all NLRP3 inhibitor compounds in development.
References:
- Teske KA et al. Cell Chem Biol 31, 349-360 e346 (2024).
- Marchetti C et al. Proc Natl Acad Sci U S A. 2018 Feb 13;115(7):E1530-E1539
(A, B) J774A.1 cells were stimulated with LPS for 4 hours (A) or overnight (B) and nigericin for 60 minutes. NLRP3 inhibitors were added before nigericin. LumitTM immunoassay was ran on whole cell culture as per manufacturer’s instructions (N=3). (C) Western blots for caspase_1 (p20 and p45) of cell lysates from J774A.1 cells stimulated with LPS and nigericin in the presence of MCC950 (MCC) and dapansutrile (OLT).
To cite this abstract in AMA style:
Tengesdal I, Marchetti C, L.Th. Jansen T, Donath M, Schlesinger N, Dinarello C. Screening NLRP3 Drug Candidates in Clinical Development:Lessons from Existing and Emerging Technologies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/screening-nlrp3-drug-candidates-in-clinical-developmentlessons-from-existing-and-emerging-technologies/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/screening-nlrp3-drug-candidates-in-clinical-developmentlessons-from-existing-and-emerging-technologies/