Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by persistent synovial inflammation, progressive joint destruction and extra-articular manifestations. Despite the advancements in the management of RA, including treat-to-target strategies and implementation of biologic (b) and targeted synthetic (ts) disease-modifying anti-rheumatic drugs (DMARDs), about 10% of RA patients remain symptomatic and develop “difficult-to-treat” (D2T) RA1. Advanced proteomic analyses of plasma offer a promising approach for monitoring disease activity and predicting RA progression.
This study aimed to profile plasma proteome in RA patients and identify proteins associated with the development of D2T RA.
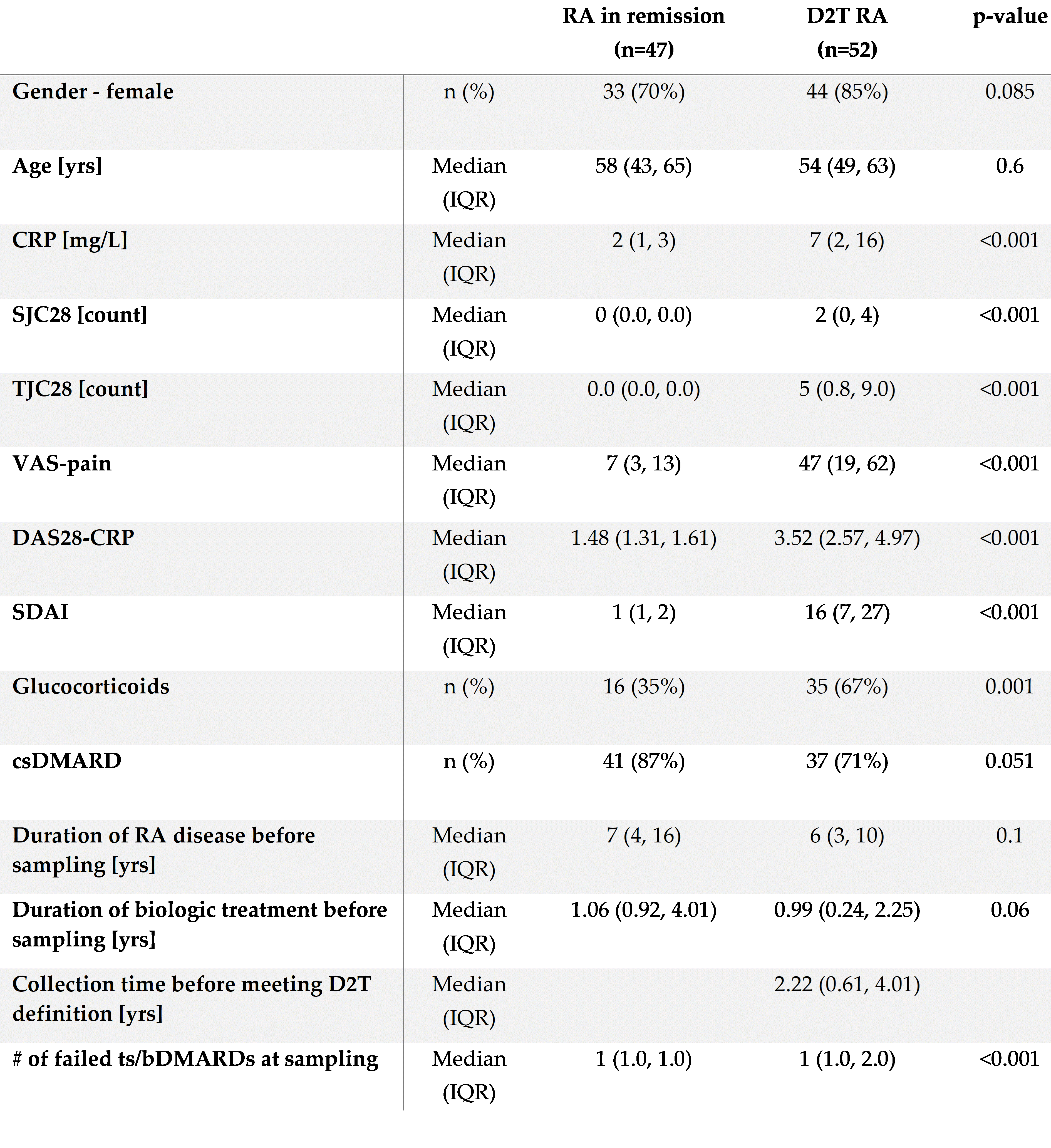
Methods: We analyzed plasma samples from 99 RA patients enrolled in the Czech biologics registry ATTRA, who began treatment with b/ts DMARDs at the Institute of Rheumatology in Prague (Table 1). The cohort consisted of 52 patients (median age 54 years, 85% females) who fulfilled the EULAR definition of D2T RA. Their plasma samples were collected 2.2 years before meeting D2T criteria. For comparison, 47 RA patients who were in sustained remission (median age 58 years, 70% females) as determined by meeting a Simplified Disease Activity Index (SDAI) < 3.3 and Swollen Joint Count (SJC) ≤ 1 over two consecutive follow-ups 12 weeks apart, were included.
We employed a kit to label 270 predesigned proteins and quantified them in the plasma using liquid chromatography with the mass-spectrometry detector. A linear regression model was employed to assess the relationship between plasma proteins and the development of D2T RA, adjusted for CRP, DAS28-ESR, HAQ and EuroQuol as significant covariates identified through prior predictive analysis.
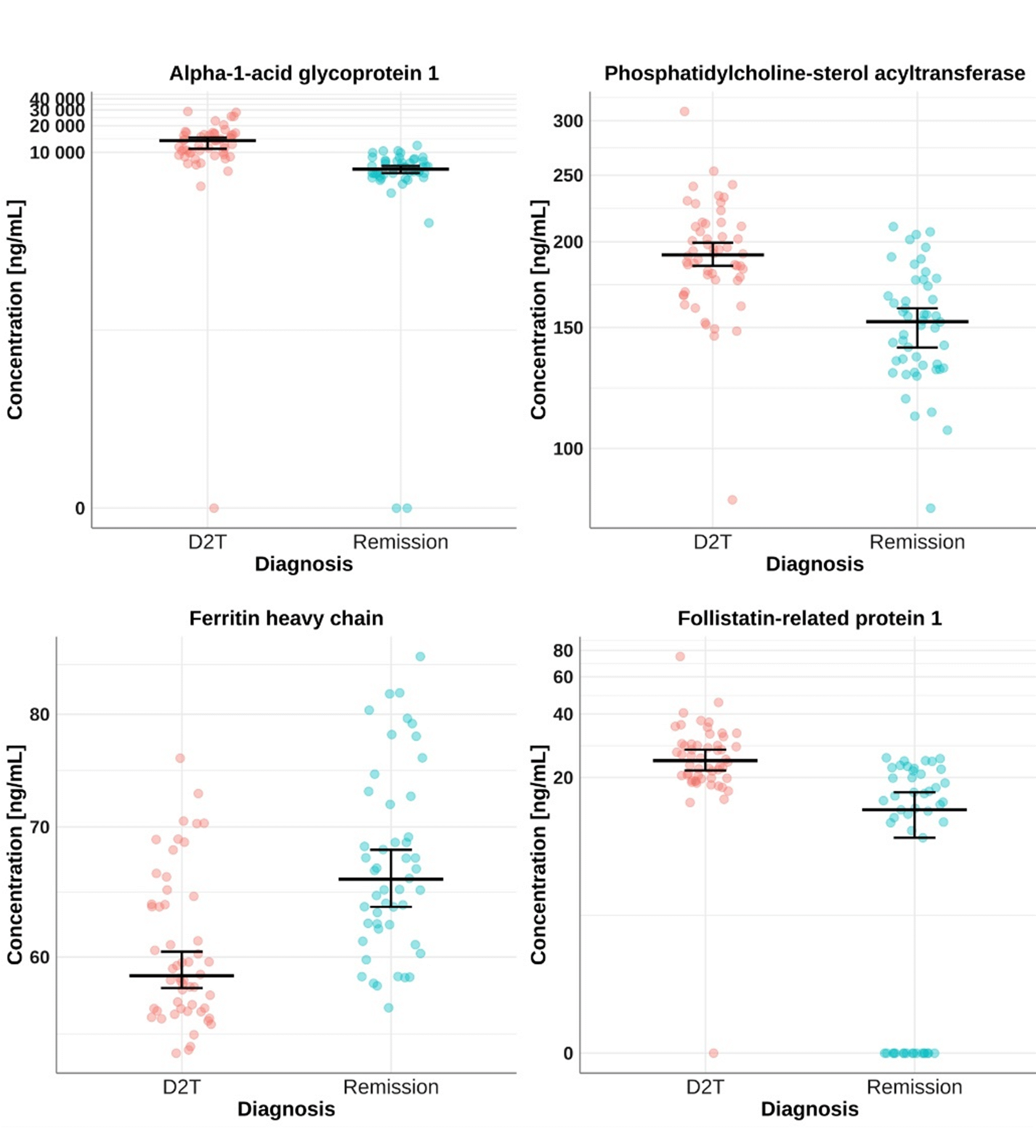
Results: Our proteome screening experiment detected 222 quantifiable miRNAs. The follow-up regression modelling revealed 12 proteins significantly associated with D2T RA compared to patients in sustained remission (p-value < 0.05, omega2 > 0.06). For example, alpha-1-acid glycoprotein 1 (1.55 fold), phosphatidylcholine-sterol acyltransferase (1.25 fold), ferritin heavy chain (1.84 fold), and follistatin-related protein 1 (1.84 fold) (Figure 1) were elevated in D2T RA patients and previously associated with RA pathophysiology.
Conclusion: Our study uncovered 12 plasma proteins associated with the development of D2T RA, with four proteins previously implicated in RA pathology showing predictive potential. Plasma proteome profiling emerges as an effective tool for disease stratification and could pave the way for tailored therapeutic strategies in the future.
Acknowledgements: Supported by SVV 260 638, BBMRI-CZ LM2023033, MHCR 023728, NU23-10-00434.
References
1. 1. Nagy G, Roodenrijs NMT, Welsing PMJ, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80(1):31-35. doi:10.1136/ANNRHEUMDIS-2020-217344
Abbreviations: CRP – C-reactive protein; SJC28 – Swollen 28-Joint Count; TJC28 – Tender 28-Joint Count; DAS28-ESR – Disease Activity Score 28-Joint Count with CRP; SDAI – Simplified Disease Activity Index; DMARDs – Disease-Modifying Anti-Rheumatic Drugs; VAS – Visual Analog Scale; cs – Conventional Synthetic; ts/b – target synthetic or biologic.
To cite this abstract in AMA style:
Mocová K, Baloun J, Prokopcová A, Brábníková Marešová K, Mann H, Vencovský J, Pavelka K, Šenolt L. Proteomic Signatures of Difficult-to-Treat Rheumatoid Arthritis: Identifying Predictive Biomarkers [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/proteomic-signatures-of-difficult-to-treat-rheumatoid-arthritis-identifying-predictive-biomarkers/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/proteomic-signatures-of-difficult-to-treat-rheumatoid-arthritis-identifying-predictive-biomarkers/