Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Baricitinib could target multiple cytokine pathways associated with juvenile idiopathic arthritis associated uveitis (JIA-U) and antinuclear antibody (ANA)-positive uveitis, providing a novel therapeutic approach. This study evaluated the efficacy and safety of baricitinib on paediatrics with active JIA-U or chronic anterior ANA-positive uveitis, with an inadequate response to methotrexate (MTX) or biologic disease modifying antirheumatic drugs (bDMARDs) and on topical corticosteroid eye drops at a stable dose.
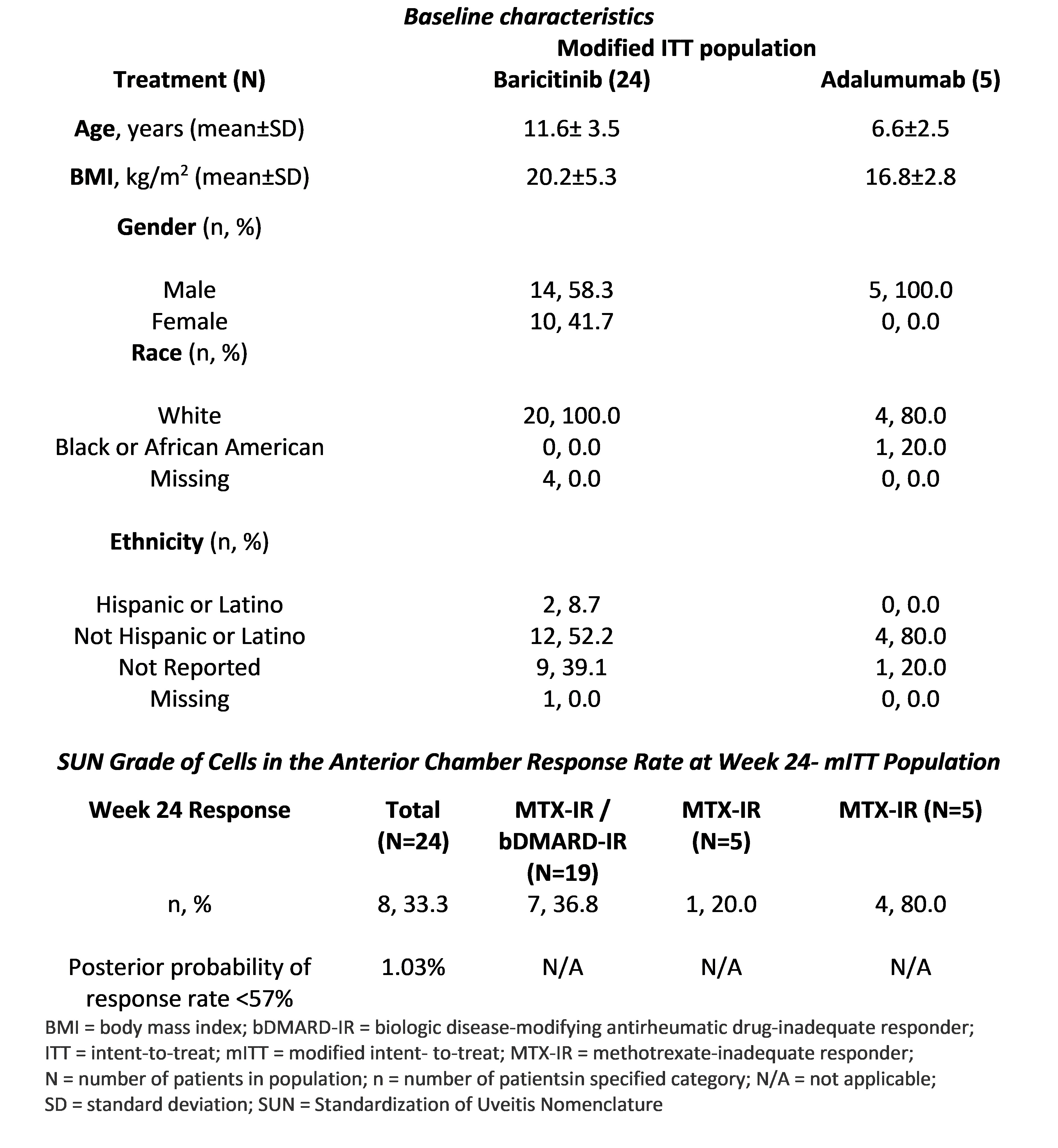
Methods: The open-label, active-controlled, Phase 3 trial, was conducted on children aged 2-< 18 years. Patients received 2mg (2-< 9 years)/4mg (9-< 18 years) oral baricitinib once daily. A reference patient group received adalimumab (subcutaneous injection) every 2 weeks (20/40mg based on weight). Primary efficacy endpoint: proportion of responders at Week 24 (W24), defined by Standardization of Uveitis Nomenclature criteria as a 2-step decrease in inflammation (anterior chamber cells) or decrease to zero through W24 in the eye most severely affected at baseline. A Bayesian analysis was performed for the primary endpoint, based on pre-specified success criteria (the posterior probability of the treatment response rate exceeding 57% is at least 80%).
Results: Baricitinib (N=24) vs Adalimumab group (N=5): mean age 11.6 vs 6.6 years. The primary endpoint of the study was not met. Baricitinib group: 33% patients achieved a response at W24, resulting in 1.03% posterior probability of a response rate of >57% (Table). The treatment-emergent adverse events (TEAEs) were consistent with the established safety profile in other baricitinib indications in paediatrics and adults. Baricitinib group: 83.3% patients reported with at least 1 TEAE (overall, 41.7% mild, 29.2% moderate and 12.5% severe).
Conclusion: The primary endpoint of the study was not met. The data provides additional information for the treatment of children with JIA uveitis refractory to both MTX and bDMARDs. Also, baricitinib safety profile in this study was consistent with previous studies in children and adults with other diseases, with most TEAEs being mild/moderate.
To cite this abstract in AMA style:
Ramanan A, Guly C, Simonini G, Keller S, Sen P, Holzkaemper T, QUARTIER P. Effectiveness and Safety of Baricitinib for the Treatment of Juvenile Idiopathic Arthritis Associated Uveitis or Chronic Anterior Antinuclear Antibody Positive Uveitis in Children [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-safety-of-baricitinib-for-the-treatment-of-juvenile-idiopathic-arthritis-associated-uveitis-or-chronic-anterior-antinuclear-antibody-positive-uveitis-in-children/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-baricitinib-for-the-treatment-of-juvenile-idiopathic-arthritis-associated-uveitis-or-chronic-anterior-antinuclear-antibody-positive-uveitis-in-children/