Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: To evaluate changes over time in fatigue measured by Patient Reported Outcome Measures Information System (PROMIS)-Fatigue scores in rheumatoid arthritis (RA) patients treated with sarilumab, and to assess the proportion of patients achieving different meaningful within-person change thresholds.
Methods: Data from the three Phase 3 randomized controlled trials of sarilumab in RA were evaluated. The 10 RA-relevant items from the 13-item Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) were scored on the PROMIS T-score metric using a pattern-scoring method. Mean changes in PROMIS-Fatigue-10a were compared, proportions of patients achieving meaningful within-person change thresholds were assessed, and results were visualized using empirical cumulative distribution function (eCDF) curves.
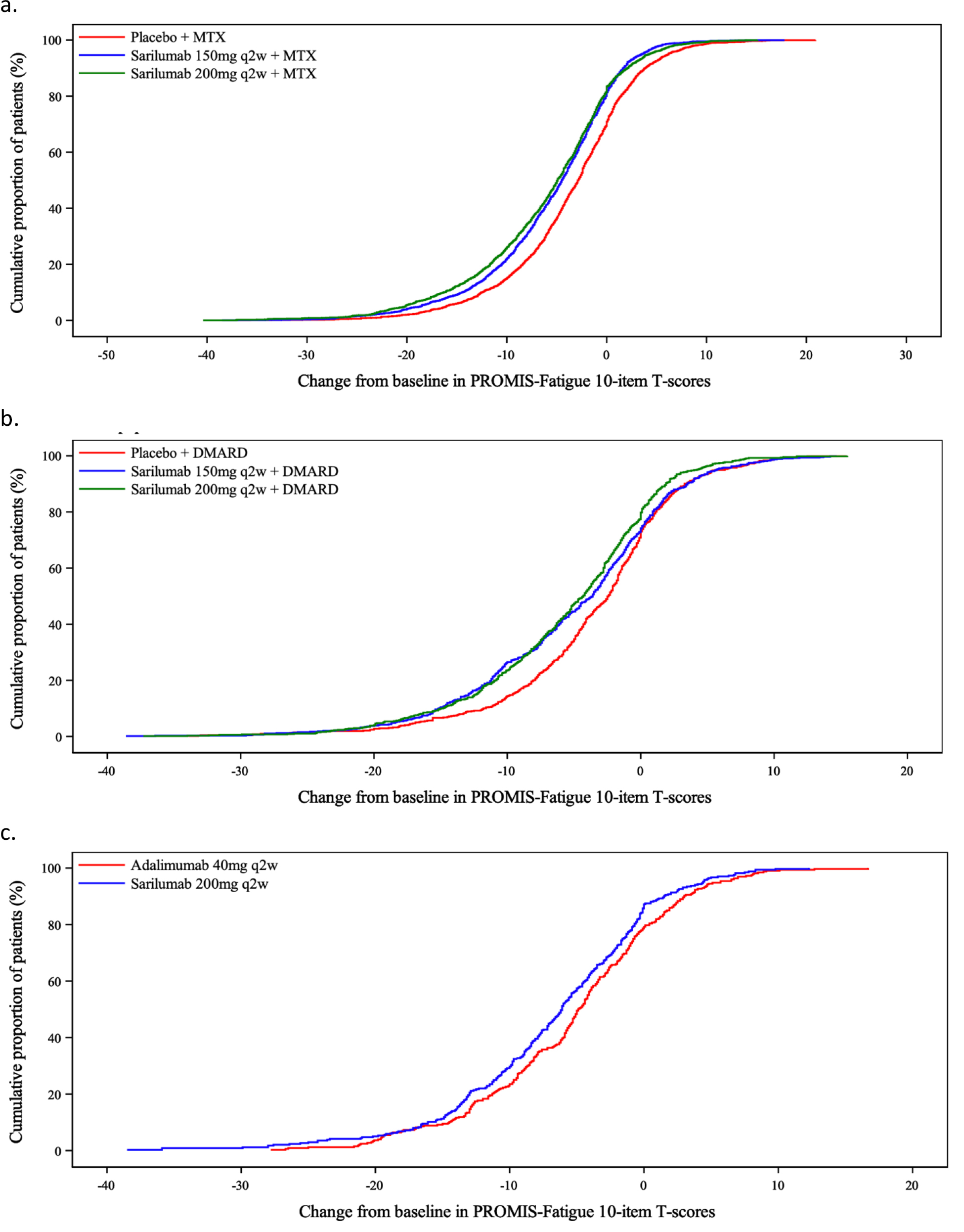
Results: RA patients reported high baseline fatigue. Using PROMIS-Fatigue-10a, at week 24, patients receiving sarilumab 150 or 200 mg every other week had rapidly improved mean levels of fatigue compared with placebo. When compared with adalimumab, patients receiving sarilumab had more improvement. eCDF curves showed separation between active treatment vs placebo with both sarilumab doses and across the score range for improvement (Figure 1). Higher proportions of patients achieved 3-, 5-, and 7-point improvements in PROMIS-fatigue in active treatment groups.
Conclusion: Substantial proportions of RA patients treated with sarilumab achieved meaningful change in fatigue measured by PROMIS-Fatigue-10a over time. This study demonstrates the convertibility of FACIT-Fatigue into the PROMIS T-score metric, the rapid reduction in fatigue with biological therapies , and the use of novel eCDF curves to show individual patient level change.
To cite this abstract in AMA style:
Bingham C, Molina E, Praestgaard A, Fiore S, Cella D. Evaluating Meaningful Within-Person Change Thresholds in PROMIS-Fatigue Scores from Three Phase 3 Clinical Trials of Sarilumab for Rheumatoid Arthritis Using Empirical Cumulative Distribution Function (eCDF) Curves [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/evaluating-meaningful-within-person-change-thresholds-in-promis-fatigue-scores-from-three-phase-3-clinical-trials-of-sarilumab-for-rheumatoid-arthritis-using-empirical-cumulative-distribution-function/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-meaningful-within-person-change-thresholds-in-promis-fatigue-scores-from-three-phase-3-clinical-trials-of-sarilumab-for-rheumatoid-arthritis-using-empirical-cumulative-distribution-function/