Session Information
Date: Saturday, November 16, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Weight loss is conditionally recommended for gout management; however, the magnitude of the effect of weight loss on incident gout and recurrent gout flares among overweight and obese individuals remains unknown. We aimed to examine the relation of the rate of weight loss induced by anti-obesity medications to the risk of incident gout and recurrent gout flares among overweight/obese individuals.
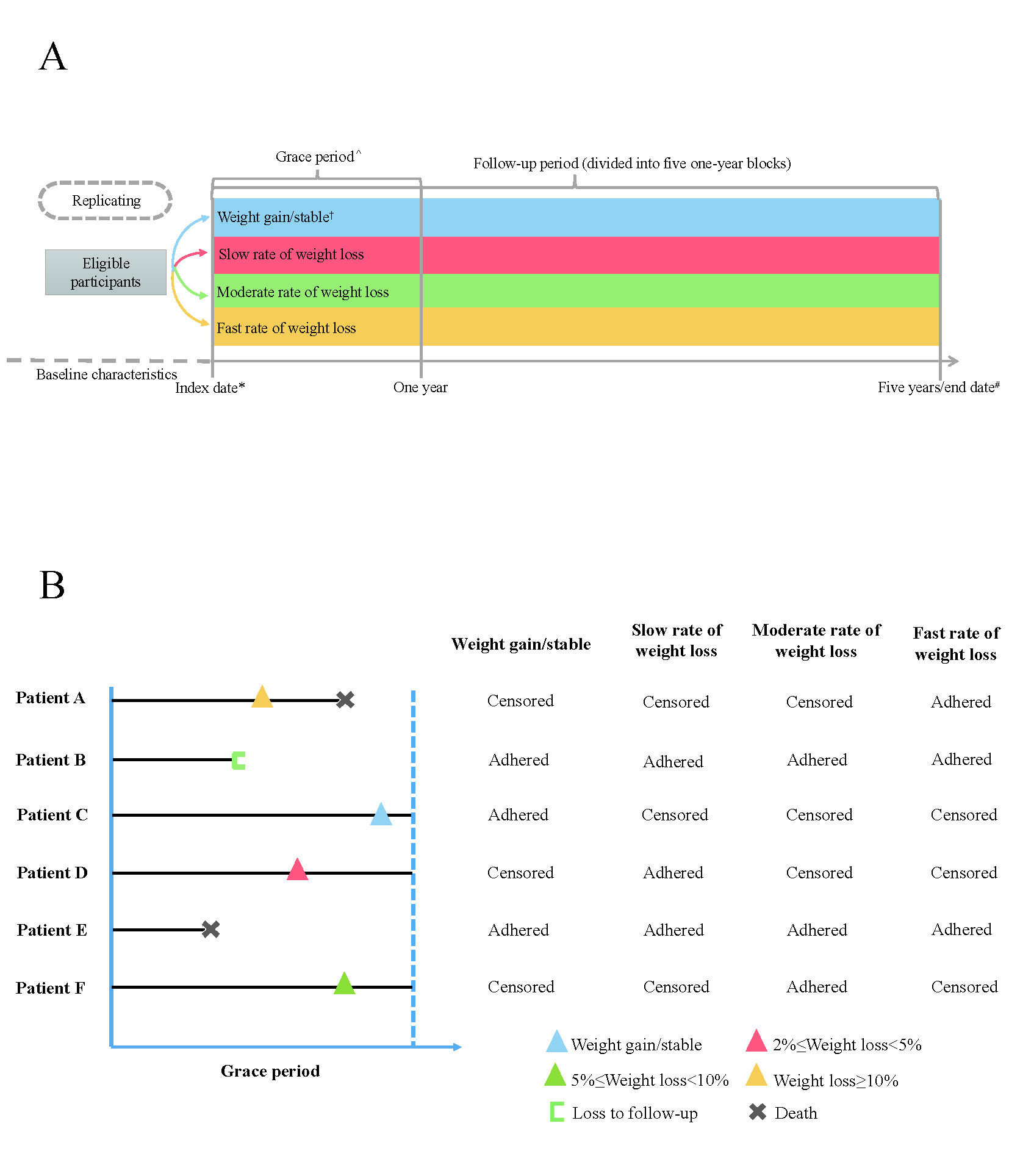
Methods: Using The Health Improvement Network database, we identified overweight or obese people aged 18 years or older initiating anti-obesity medications. We emulated analyses of a hypothetical target trial to assess the effect of slow (2-5%), moderate (5-10%) or fast (≥10%) rate of weight loss induced by the anti-obesity medications within 1-year on incident gout and recurrent gout flares over 5-year follow-up, respectively (Figure 1).
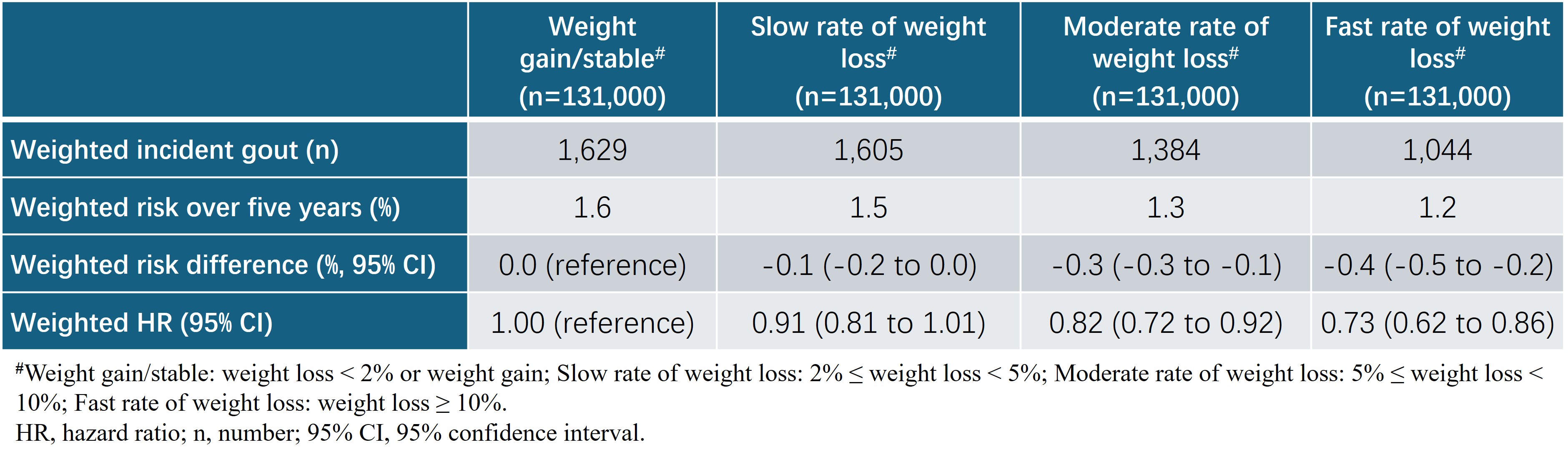
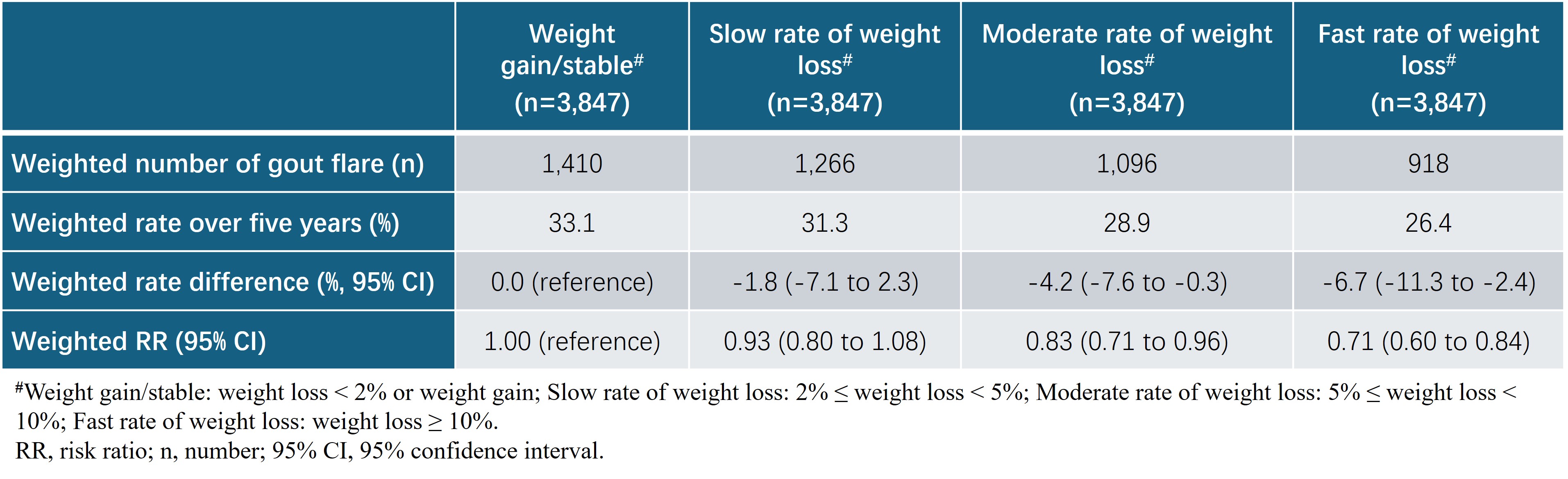
Results: Among 131,000 participants without gout and initiating orlistat, the 5-year risk of incident gout was 1.6%, 1.5%, 1.3%, and 1.2% for weight gain/stable, slow, moderate and fast rate of weight loss arms, respectively. Compared with the weight gain/stable arm, the hazard ratios were 0.91 (95% confidence interval [CI]: 0.81 to 1.01), 0.82 (95%CI: 0.72 to 0.92), and 0.73 (95%CI: 0.62 to 0.86) for slow, moderate and fast rate of weight loss arms, respectively (Table 1). Similar results were observed for the recurrent gout flares among 3,847 overweight or obese people with gout initiating orlistat (Table 2).
Conclusion: A higher rate of weight loss induced by orlistat within 1-year was associated with lower risks of incident gout and lower rates of recurrent gout flares among overweight or obese people.
*Index date: the date of orlistat initiation.
#End date: the date of incident gout, death, disenrollment from a GP practice participating in THIN, 5 years of follow-up, or the end of the study (30 June 2023), whichever occurred first.
†Weight change = (weightlast record during the grace period –weightbaseline)/weightbaseline*100%. We used the following cut points to assign categories of weight change: Weight gain/stable (<2% weight loss or weight gain), slow (≥2 to <5% loss), moderate (≥5 to <10% loss) or fast (≥10%) rate of weight loss.
^Grace period: participants were given one-year to reduce their weight after initiating with the orlistat. Replicates would be censored if they deviate from their assigned treatment at the end of the grace period (e.g., replicate in the fast rate of weight loss group (≥10% loss) would be censored if the participant’s weight loss <10% or weight gain).
GP, general practitioner; THIN, The Health Improvement Network.
To cite this abstract in AMA style:
Wei J, Wang Y, Dalbeth N, Xie J, Wu J, Zeng C, Lei G, Zhang Y. Weight Loss Induced by Anti-obesity Medications and Gout Among Overweight and Obesity Individuals: A Population-based Cohort Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/weight-loss-induced-by-anti-obesity-medications-and-gout-among-overweight-and-obesity-individuals-a-population-based-cohort-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/weight-loss-induced-by-anti-obesity-medications-and-gout-among-overweight-and-obesity-individuals-a-population-based-cohort-study/