Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Axial Spondyloarthritis is characterized by both inflammation and new bone formation. Current therapeutic targets aim to control inflammation, however, over 40% of patients do not respond to them. The novel deubiquitinase molecule TRABID has been shown to epigenetically control the expression of IL12/23 in the experimental autoimmune encephalomyelitis mouse model. Furthermore, our group has previously shown significantly increased expression of TRABID in human AxSpA tissues. We have also shown that the inhibition of TRABID in monocyte and osteoblast cell culture models significantly suppresses cytokine production and bone mineralization respectively. Therefore, this study aims to understand the in-vivo therapeutic potential of TRABID inhibition by the small molecule inhibitor NSC112200 in the SKG pre-clinical mouse model of AxSpA.
Methods: 8-week-old SKG mice were injected with the disease-causing agent curdlan or a PBS control. 8 weeks after disease induction, mice were sacrificed, and their tissues were harvested. Immunohistochemistry was used to determine the expression of TRABID in the affected joints (ankle and tail). Immune cells from the spleen were also harvested and incubated with the TRABID small molecule inhibitor NSC112200 and lipopolysaccharide to induce cytokine production for 24 hours. ELISA was then used to determine the production of TNFa in the cell culture supernatant. Finally, we wanted to determine if inhibiting TRABID in-vivo could be potentially therapeutic. 8-week-old SKG mice were injected with curdlan and 3 doses of NSC112200 at varying intervals for 8 weeks: low-dose (150mM/kg body weight every 2 days), mid-dose (300mM/kg every 5 days) and high-dose (300mM/kg every 2 days). Arthritis severity was scored every week. 8 weeks after disease induction, mice were sacrificed and tissues were harvested for histopathological analysis, nanostring immunology profiling and immunohistochemistry.
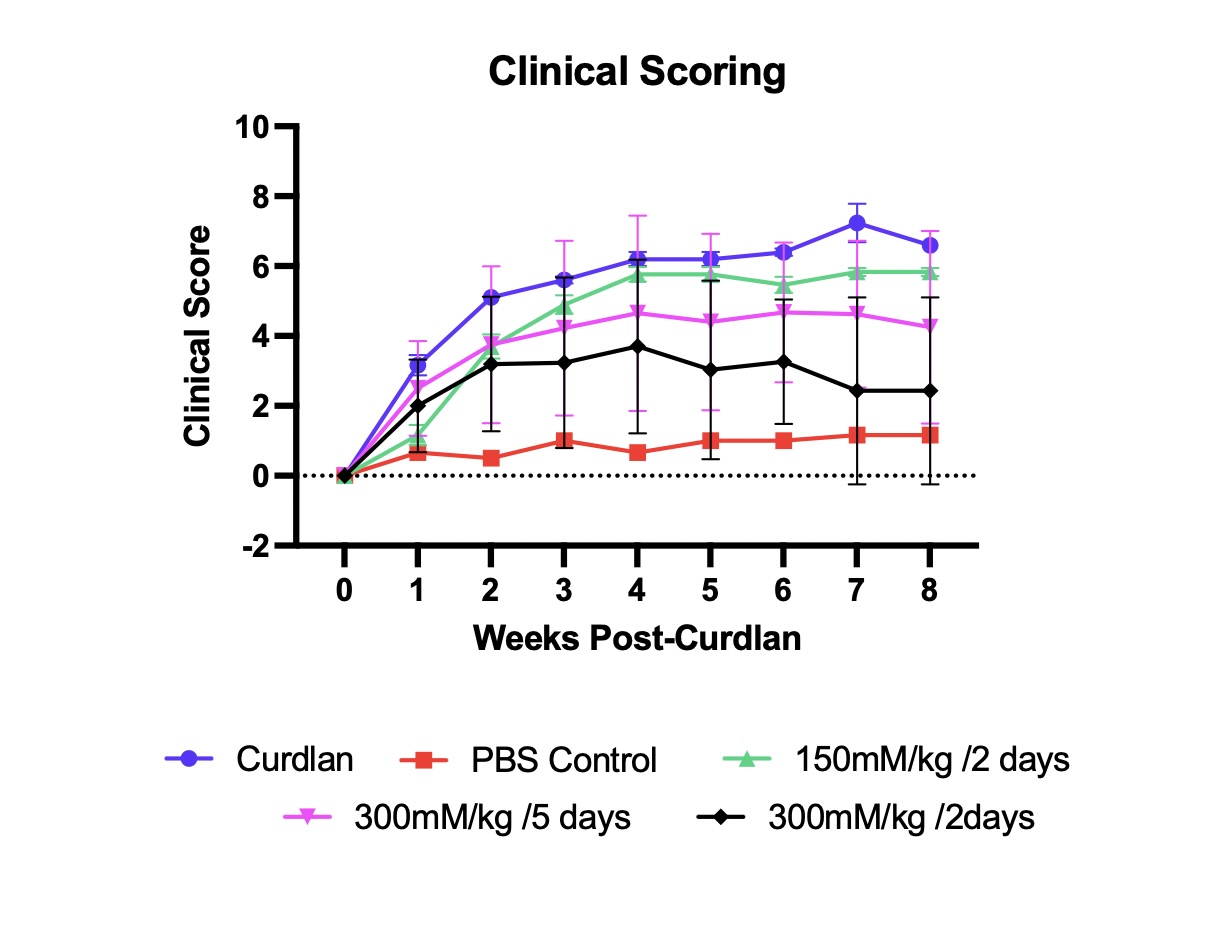
Results: TRABID was significantly upregulated in curdlan disease-induced mice (n=6) in the ankle (p=0.02) and tail (p=0.02) compared to controls (n=3). Moreover, splenocytes incubated with NSC112200 showed a marked dose-dependent decrease in TNFa production (p< 0.0001). Finally, after 8 weeks of treatment with NSC112200 at the 3 indicated doses, mice showed a dose dependant decrease in disease severity (p< 0.0001), including symptoms of blepharitis, paw swelling and psoriasis of the ears, snout and tail (Figure 1). Additionally, histopathological analysis of the ankle and tail showed decreases in immune infiltrate in the bone marrow, synovium and Achilles tendon, and decreased nucleus pulposus degeneration in the tail spine (p=0.023).
Conclusion: All together our data suggests that increased TRABID expression contributes to the pathology of disease in curdlan-treated SKG mice. Inhibiting the function of TRABID using NSC112200 showed therapeutic potential in these mice by possibly controlling the expression of key disease-causing cytokines such as TNFa. While the exact cellular and genetic targets of this inhibition are currently under exploration, this data provides early proof for a novel therapeutic target that can be blocked to control inflammation.
To cite this abstract in AMA style:
Srinath A, Mauro D, Korshko M, Foroozan S, Aparnathi M, Ciccia F, Haroon N. Inhibition of the Deubiquitinase TRABID in a Pre-clinical Mouse Model of Axial Spondyloarthritis Decreases Disease Severity [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/inhibition-of-the-deubiquitinase-trabid-in-a-pre-clinical-mouse-model-of-axial-spondyloarthritis-decreases-disease-severity/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/inhibition-of-the-deubiquitinase-trabid-in-a-pre-clinical-mouse-model-of-axial-spondyloarthritis-decreases-disease-severity/