Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 7:30AM-9:00AM
Background/Purpose: In osteoarthritis (OA) of the knee, intraarticular injections of corticosteroids can relieve pain, reduce inflammation, and improve mobility, but the effect is not predictable, and the duration of pain relief can be short. The benefit of repeat injection has not been established. TLC599 is a liposomal formulation of dexamethasone sodium phosphate (DSP) intended for administration by local injection to provide sustained relief of pain of OA of the knee. The current study was conducted to confirm earlier phase 2 results demonstrating pain relief in OA patients over 24 weeks (Hunter et al., 2022), and to study the benefit of repeat injection over one year.
Methods: The study was a Phase 3, randomized, double-blinded, 3-armed, placebo- and active-controlled study to evaluate the efficacy and safety of TLC599 in single or repeat doses, in patients with K-L Grade 2-3 OA of the knee and average daily pain (ADP) from screening diary completion in the index knee of 5-9 (on a scale of 0-10). A total of 506 patients were randomized in a 2:1:1 ratio to receive an injection of TLC599 12 mg, DSP 4 mg, or saline placebo. At Week 24, eligible patients in the TLC599 and placebo arms received a second blinded injection of the same treatment, while patients in the DSP arm received a blinded injection of TLC599. Efficacy and safety were assessed through Week 52. Among other efficacy parameters, ADP and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain were assessed using Mixed Models for Repeated Measures (MMRM) and Analysis of Covariance (ANCOVA), respectively.
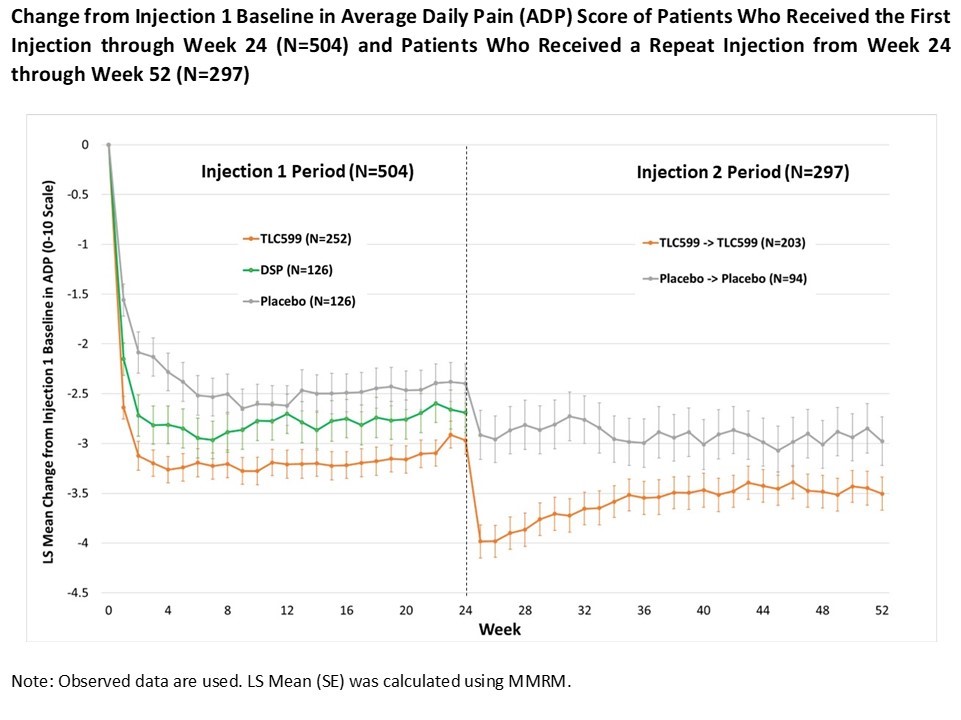
Results: With respect to WOMAC pain, TLC599 was numerically superior to placebo at all time points through week 24, and statistically superior (p< 0.05) at Week 12, the primary endpoint. For ADP, TLC599 was numerically and statistically superior (p< 0.05) to placebo at all time points during Injection 1 period (Figure).Additionally, at Week 12, the reduction in ADP for TLC599 was superior to DSP (p< 0.05). A total of 203 patients who received an initial injection of TLC599 and 94 patients who received an initial injection of placebo were eligible and received repeat doses of TLC599 or placebo. The mean reduction in ADP from the injection 1 baseline for TLC599 was numerically superior to placebo at all time points through Week 52 and statistically superior through Week 34. TLC599 was generally well tolerated, with the number and type of adverse events similar among all three treatment groups. At Weeks 1 and 25 (after the second injection), there was a transient reduction in mean morning serum cortisol levels in patients who received TLC599, but this returned to normal by Weeks 2 and 26. There were no signs or symptoms of adrenal insufficiency in any patient.
Conclusion: TLC599 demonstrated a benefit over placebo in ADP and WOMAC pain in patients with OA of the knee after a single injection that was sustained to 24 weeks.A second injection of TLC599 at Week 24 provided further benefit to Week 52. These results provide additional information about the benefits of repeat steroid injection in OA of the knee and suggest that TLC599 may provide prolonged benefit and offer an alternative treatment for the management of OA knee pain.
To cite this abstract in AMA style:
Spencer-Green G, Hunter D, Schnitzer T, Shih S, Tai T, Kao C, Huang S. A Phase 3 Study of Repeat Injection of TLC599 in Osteoarthritis of the Knee: Benefits to 52 Weeks [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-phase-3-study-of-repeat-injection-of-tlc599-in-osteoarthritis-of-the-knee-benefits-to-52-weeks/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-3-study-of-repeat-injection-of-tlc599-in-osteoarthritis-of-the-knee-benefits-to-52-weeks/