Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes IV: Outcomes & Comorbidity
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
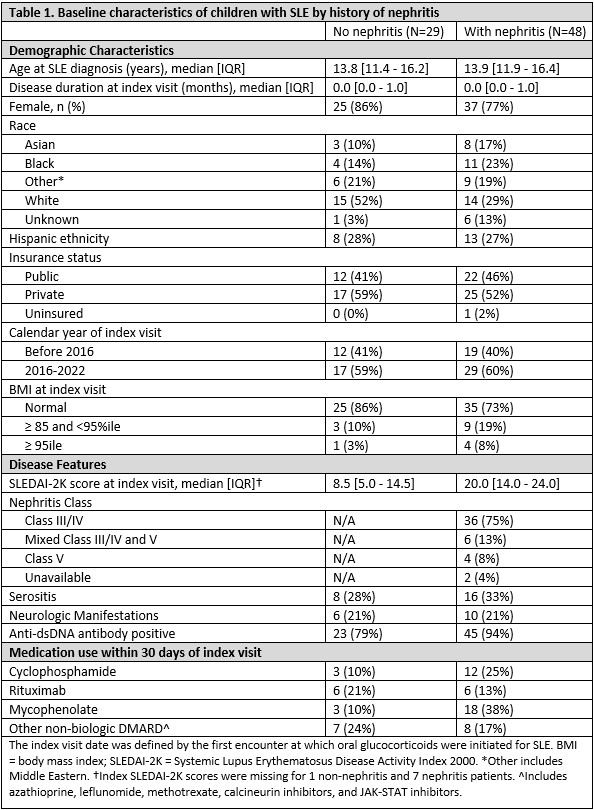
Background/Purpose: Chronic glucocorticoid (GC) morbidity is rarely captured as a standardized clinical outcome in pediatric rheumatic conditions. The pediatric glucocorticoid toxicity index (pGTI) (Brogan et al. 2022) is a new, pediatric-specific tool to measure GC toxicity in research and clinical care. Common clinically important toxicities are organized into domains and weighted according to severity. We used the pGTI to quantify and identify risk factors for GC toxicity in children with pediatric-onset systemic lupus erythematosus (pSLE) with or without lupus nephritis (LN).
Methods: We conducted a single-center cohort study of patients with pSLE, stratified by LN. We included patients with rheumatology visits since January 2016 and ³6 months of systemic GC treatment and followed them through March 2023. We scored change in GC toxicity using a modified version of the pGTI, including steroid toxicity items in 12/15 subdomains, at visits every 6 months (+/-2) for up to 3 years. Glucose metabolism, dyslipidemia, and bone density domains could not be assessed due to sparse data. Index date was the visit at which GC were initiated. Positive scores indicate new/worsening toxicity and negative scores indicate improvement. We also calculated the Cumulative Worsening Score (CWS), an endpoint of the pGTI representing a continuous summation of any GC toxicity accrued over time. Mixed effects linear regression was used to estimate associations of baseline factors and GC exposure with CWS over time.
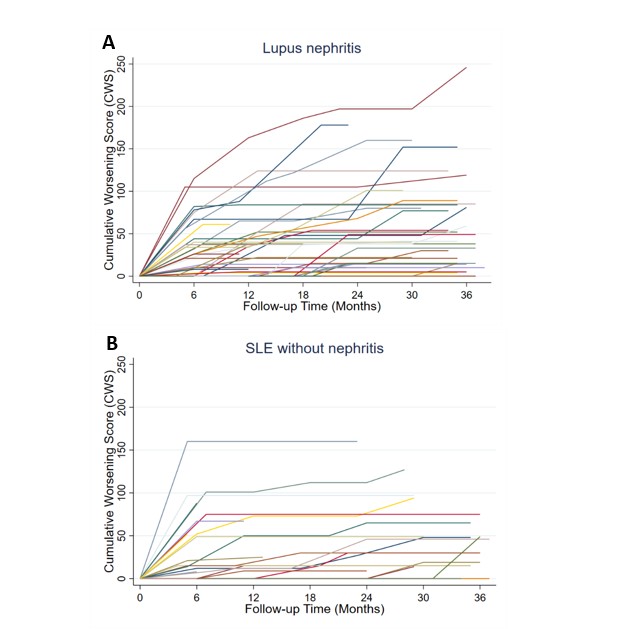
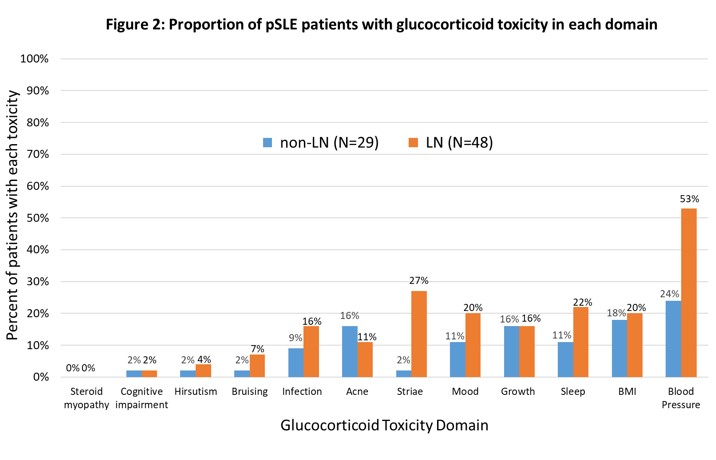
Results: We included 425 visits by 77 pSLE patients (median 6 visits/patient [IQR 5-7]), of which 48 had LN (Table 1). In both groups, the most common toxicity was worsening blood pressure; this was more pervasive in the LN group (53% vs. 24% of patients), which also exhibited greater damage (24% vs. 8% developed hypertensive emergency or posterior reversible encephalopathy syndrome). Increased body mass index (BMI), sleep disturbance, and growth delay were also common. Compared to LN patients, those without LN had similar or less frequent toxicity in every subdomain except for acne (Fig 2). Median CWS at month 12 was 15 (IQR [0-47]; N = 47) in the LN subgroup and 15 (IQR [0-67]; N = 22) in those without LN. 46% of patients with LN and 34% without accrued new/worsening toxicity beyond 12 months (Fig 1).
Cumulative dose associated with greater CWS (1.5 unit increase in CWS for every 1000 mg higher cumulative prednisone dose, 95% CI [0.03 – 2.8]), adjusted for time, but there was large between-subject variance. Of baseline factors in Table 1, younger age of onset, need for initial treatment with rituximab, and baseline BMI >95th%ile were significantly associated with higher CWS over time.
Conclusion: Patients with pSLE exhibit significant GC toxicity captured by the pGTI, particularly in blood pressure, BMI, sleep, and growth subdomains. This cohort also demonstrated substantial between-subject variability in toxicity. We will further assess the construct validity and feasibility of the pGTI in upcoming multicenter analyses. Standardized, pediatric-specific assessment of GC toxicity may help facilitate reduction in GC-associated morbidity in children with SLE.
To cite this abstract in AMA style:
Zhang E, Capponi S, Scobell R, Alonzi G, Hlobik M, Meidan E, Lo M, Halyabar O, Hazen M, Cohen E, Henderson L, Case S, Chang M, Daga A, Hausmann J, Bakhsh A, Kim L, Ibanez D, Wobma H, Dedeoglu F, Sundel R, Nigrovic P, Costenbader K, Son M, Chang J. Real-World Application of the Pediatric Glucocorticoid Toxicity Index in Children with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/real-world-application-of-the-pediatric-glucocorticoid-toxicity-index-in-children-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-application-of-the-pediatric-glucocorticoid-toxicity-index-in-children-with-systemic-lupus-erythematosus/