Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: PD-1 immune checkpoint inhibitors are able to re-activate restrained anti-tumor T-cell responses in melanoma patients. However, PD-1 inhibition may also activate auto-reactive T-cells leading to immune-related adverse events (irAEs). Autoantibodies might also play a role in anti-PD-1 induced irAEs and could potentially be used to identify patients at risk of developing these irAEs. Therefore, we investigated the association between autoantibody-positivity and treatment toxicity and response in melanoma patients treated with anti-PD-1.
Methods: This multicenter, retrospective study included 143 advanced melanoma patients treated with anti-PD-1. Until six months after treatment initiation, toxicities grade ≥2 and recurrences/responses were captured. Autoantibody measurements, comprising IgM rheumatoid factor (RF), antinuclear antibodies (ANA), anti-cyclic citrullinated peptide antibodies (anti-CCP2), anti-thyroid peroxidase (anti-TPO) and anti-thyroglobulin (anti-Tg) were performed in an ISO15189 accredited laboratory at baseline and after three months of treatment.
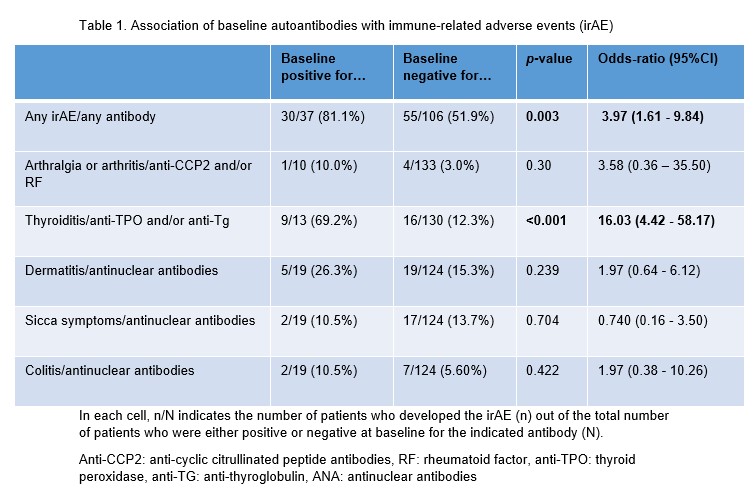
Results: A total of 169 irAEs were experienced by 86 patients, the most common being thyroiditis (n=25), dermatitis (n=24), and sicca complaints (n=19). More irAE (30/37 [81%]) were observed in patients with pre-existing detectable autoantibodies than in patients without these autoantibodies (55/106 [52%], p = 0.003) (table 1). This was mainly due to the strong association of anti-thyroid antibodies and thyroid dysfunction (69% in anti-TPO and/or anti-Tg positive patients versus 12% in anti-TPO and/or anti-Tg negative patients, p < 0.001). No association between anti-CCP2, RF or ANA and arthritis, dermatitis, sicca or colitis was observed. The association with thyroid antibodies was strongest in female patients: of seropositive female patients 7/7 (100%) developed thyroiditis, compared to only 10/48 (21%) of anti-TPO and anti-Tg negative patients.
Seroconversion during anti-PD-1-treatment was only seen for thyroid antibodies. Here the combined positivity for anti-TPO and/or anti-Tg increased from 13/143 (9.1%) at baseline to 24/143 (17%) on treatment (p = 0.003). For the other autoantibodies the total number of seropositive patients stayed stable or decreased on treatment (table 2). Seroconversion of anti-Tg and/or anti-TPO was associated with thyroid dysfunction in female patients: 6/10 [60%] of seroconverted patients developed thyroid dysfunction compared to 3/38 [8%] patients that remained seronegative (p = 0.001). Autoantibody-positivity was not associated with disease recurrence (p = 0.45) or response (p = 0.69).
Conclusion: Autoantibody-positivity prior to anti-PD-1 therapy is associated with the development of irAEs in melanoma patients, indicating that anti-PD-1 therapy not only leads to loss of tolerance to tumor antigens but also to nontumor autoantigens. Baseline positivity and seroconversion of anti-thyroid antibodies were associated with the development of thyroiditis.
In each cell, n/N indicates the number of patients who developed the irAE (n) out of the total number of patients who were either positive or negative at baseline for the indicated antibody (N).
Anti-CCP2: anti-cyclic citrullinated peptide antibodies, RF: rheumatoid factor, anti-TPO: thyroid peroxidase, anti-TG: anti-thyroglobulin, ANA: antinuclear antibodies
Anti-CCP2: anti-cyclic citrullinated peptide antibodies, RF: rheumatoid factor, anti-TPO: thyroid peroxidase, anti-TG: anti-thyroglobulin, ANA: antinuclear antibodies
To cite this abstract in AMA style:
van Wesemael T, Borgers J, Gelderman K, Verdegaal E, Korse C, Welters M, Kapiteijn E, van Houdt W, van der Burg S, Toes R, Thienen J, Haanen J, van der Woude D. Autoantibody-positivity Before Treatment Is Associated with Immune-related Adverse Events in Melanoma Patients Treated with anti-PD-1 [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/autoantibody-positivity-before-treatment-is-associated-with-immune-related-adverse-events-in-melanoma-patients-treated-with-anti-pd-1/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autoantibody-positivity-before-treatment-is-associated-with-immune-related-adverse-events-in-melanoma-patients-treated-with-anti-pd-1/