Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Lack of timely referral of suspected axSpA patients to rheumatologists contributes to misdiagnosis, delayed treatment, and poor outcomes. Currently there are no formal guidelines in the United States (US) to guide which chronic back pain (CBP) patients should be referred to rheumatology for evaluation of axSpA. To synthesize the evidence for the Spondyloarthritis Research and Treatment Network (SPARTAN) 2023 referral recommendations, we performed a systematic literature review (SLR) and meta-analysis to calculate predictive values of spondyloarthritis (SpA) features.
Methods: We generated Population, Intervention, Control and Outcome (PICO) questions using the framework “Are patients with CBP with SpA feature XYZ more likely to be diagnosed (or classified) as axSpA compared to those without SpA feature XYZ?” The list of SpA features was developed with input from research team members as well as stakeholders including patient partners. Databases including Cochrane Library, Google Scholar, Ovid Embase, Ovid Medline, PubMed, Scopus, and Web of Science Core Collection were searched from inception to April 2022 using relevant keywords to find observational studies in which CBP patients were referred to rheumatologists with suspicion of axSpA and which included information about SpA features in those diagnosed with axSpA and comparators. We followed PRISMA guidelines to report the SLR findings. Two reviewers from a team of 13 reviewers screened abstracts and full-length articles and abstracted the data from eligible studies. Conflicts were resolved by a senior author. Risk of bias was assessed using the QUADAS2 tool. A meta-analysis was performed to calculate and pool sensitivity, specificity, positive likelihood ratios (LR+), and positive predictive values for each SpA feature.
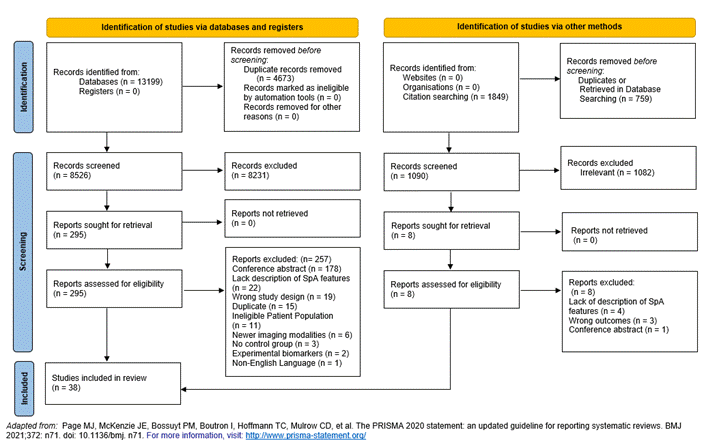
Results: We screened the title and abstracts of 8526 articles and 295 full-length articles were selected for full text review. Thirty-eight full text publications were included for data abstraction and evidence synthesis (Figure 1). Table 1 shows the test characteristics of 28 individual SpA features. LR+ ranged from 0.5 to 10. Objective SpA features including imaging evidence of sacroiliitis (6.4), elevated CRP (3.77) and positive HLA B27 (4.20) had higher LR+ followed by uveitis (3.25) and family history of spondyloarthritis (2.32). Except for good response to NSAIDs (2.32), individual items of inflammatory back pain as well as peripheral inflammatory arthritis, psoriasis, enthesitis had LR+ less than 2.
Conclusion: Our SLR and meta-analysis reveal important information about predictive values of individual SpA features and provide a foundation for formulating evidence-based referral recommendations in a data-driven process –first such effort in North America. The ultimate goal of this endeavor is to facilitate early and appropriate referral of patients with suspected axSpA to rheumatologists. Low LR+ for individual SpA features may necessitate using combinations of SpA features in the referral strategy.
To cite this abstract in AMA style:
Danve A, Dubreuil M, Alexander S, afinogenova Y, Bittar M, Grimshaw A, Fraenkel L, LaValley M, Kumthekar A, liew J, Magrey M, Majithia V, Merjanah S, Norton H, Walsh J, Deodhar A. Systematic Literature Review and Meta-analysis Informing the Development of 2023 Spondyloarthritis Research and Treatment Network (SPARTAN) Referral Recommendations for Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/systematic-literature-review-and-meta-analysis-informing-the-development-of-2023-spondyloarthritis-research-and-treatment-network-spartan-referral-recommendations-for-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/systematic-literature-review-and-meta-analysis-informing-the-development-of-2023-spondyloarthritis-research-and-treatment-network-spartan-referral-recommendations-for-axial-spondyloarthritis/