Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is a leading cause of mortality in RA with limited treatment options as well as lack of available animal modeling systems for preclinical investigations. We previously demonstrated that co-exposure of the collagen induced arthritis (CIA) with repetitive airborne lipopolysaccharide (LPS) exposure leads to increased autoimmunity, arthritis, and pro-fibrotic/pro-inflammatory lung disease. In addition to recapitulating important histopathologic features of RA-ILD, this model demonstrates more severe arthritis and lung manifestations in male mice. Platelet-derived growth factor (PDGF)-BB and its receptor (PDGFR) are important cellular proliferation and growth with dysregulation associated with cancers and tissue fibrosis, with limited and conflicting studies in RA. In the present study, we leveraged our novel mouse preclinical animal modeling system to investigate the potential role of PDGF-BB/PDGFR, in RA-associated lung disease.
Methods: Arthritis-prone male (DBA/1J) mice (n=5/group) received either: Sham (saline injection/inhalation), CIA (CIA on day 1 and 21/saline inhalation), LPS (saline injection/ LPS 100ng inhalation), or CIA+LPS (CIA injection/LPS inhalation) for 5 weeks. At week 5, serum was collected for quantification of PDGF-BB using ELISA in all 4 groups. Based on marked serum differences observed between CIA+LPS and Sham, lung tissues were isolated for quantification of PDGF-BB and PDGF-receptor expression using fluorescent immunohistochemistry (IHC) in these two treatment groups. Serum PDGF-BB levels were compared using one-way ANOVA and Bonferroni multiple comparison tests across the 4 treatment groups. Fluorescent IHC, expressed in mean pixel density, was quantified and compared in CIA+LPS vs. Sham-treated mice using a t-test.
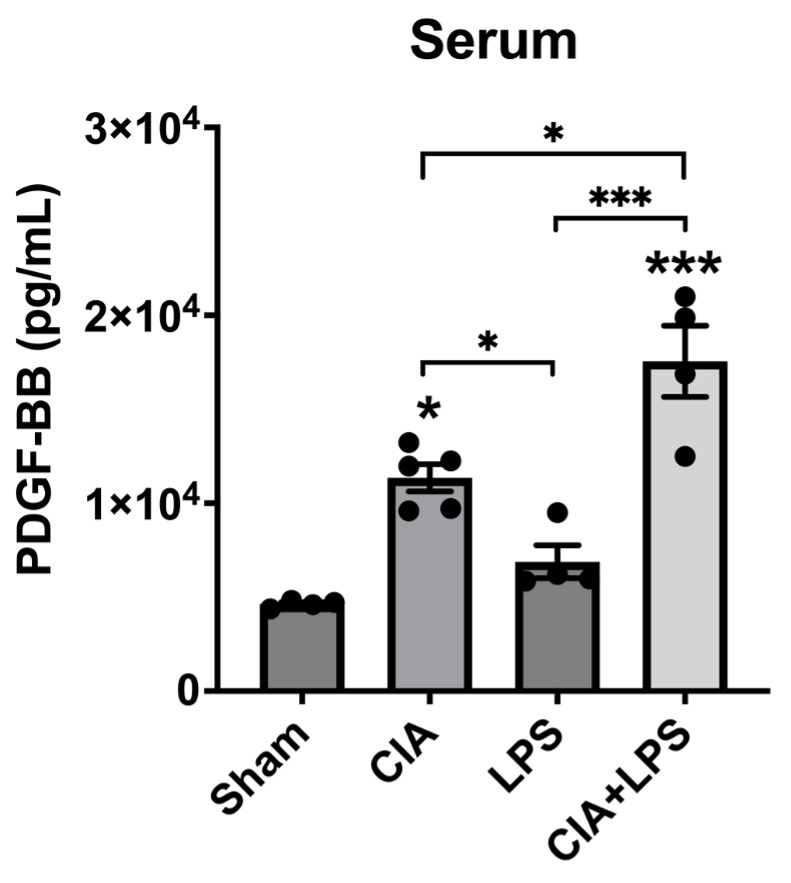
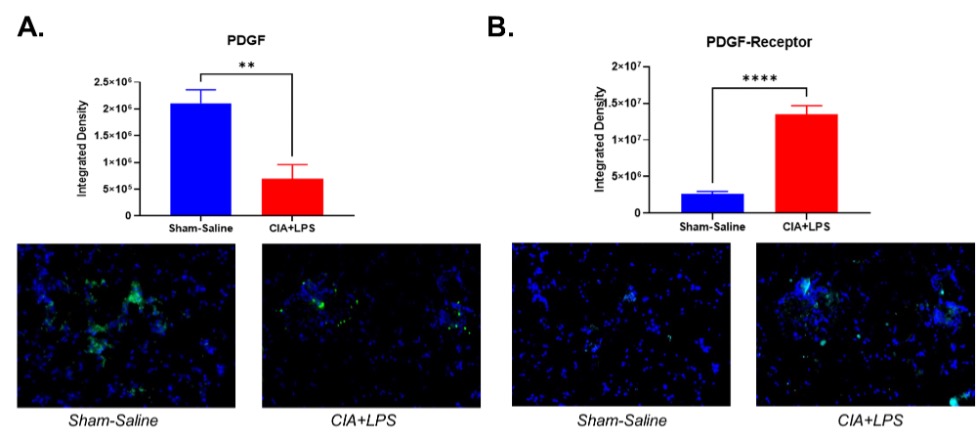
Results: Serum PDGF-BB concentrations (pg/ml mean ± SEM) were the highest in the CIA+LPS group (1.6×104 ± 1.2×103; p< 0.05 vs. all others) (Figure 1). Also, serum PDGF-BB levels were significantly increased in CIA group (1.1×104 ± 5.5×102) compared to Sham (4.7×103 ± 1.1×102; p< 0.05) and LPS (6.9×103 ± 8.8×102; p< 0.05) groups. Staining of lung tissues demonstrated that PDGF-BB expression was decreased significantly in CIA+LPS compared to Sham (p< 0.01) (Figure 2). In contrast, PDGF-receptor expression was increased significantly in CIA+LPS (p< 0.0001) compared to Sham.
Conclusion: Co-exposure of mice to CIA+LPS profoundly increased circulating PDGF-BB concentrations. In the corresponding lung tissues of CIA+LPS mice, PDGF-BB expression was strikingly reduced while PDGF-receptor expression was increased. These findings imply that dual exposure modulate the PDGF-BB/PDGFR pathway that release and/or production of PDGF is altered. Moreover, these observations suggest that PDGF-BB may serve as an important mediator of the inflammatory and fibrotic processes underlying RA-ILD pathogenesis and could represent a potential novel target for treatment.
To cite this abstract in AMA style:
Aripova N, Duryee M, Nelson A, Hunter C, Butler B, England B, Poole J, Thiele G, Mikuls T. Platelet-Derived Growth Factor Is a Mediator of Disease in an Animal Model of Rheumatoid-Associated Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/platelet-derived-growth-factor-is-a-mediator-of-disease-in-an-animal-model-of-rheumatoid-associated-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/platelet-derived-growth-factor-is-a-mediator-of-disease-in-an-animal-model-of-rheumatoid-associated-interstitial-lung-disease/