Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Pediatric Rheumatology – Clinical II: Connective Tissue Disease
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Juvenile systemic sclerosis (jSSc) is an orphan disease with a prevalence of 3 in 1,000,000 children. In adult patients there are significant differences between clinical presentation of cutaneous diffuse (djSSc) and cutaneous limited phenotypes(ljSSc).
Methods: We reviewed the baseline clinical characteristics of the patients, who were recruited to the jSScC prior to April 2023. jSScC is a prospective cohort of jSSc patients, who developed the first non-Raynaud´s symptom before the age of 16 years and were under the age of 18 years at the time of inclusion.
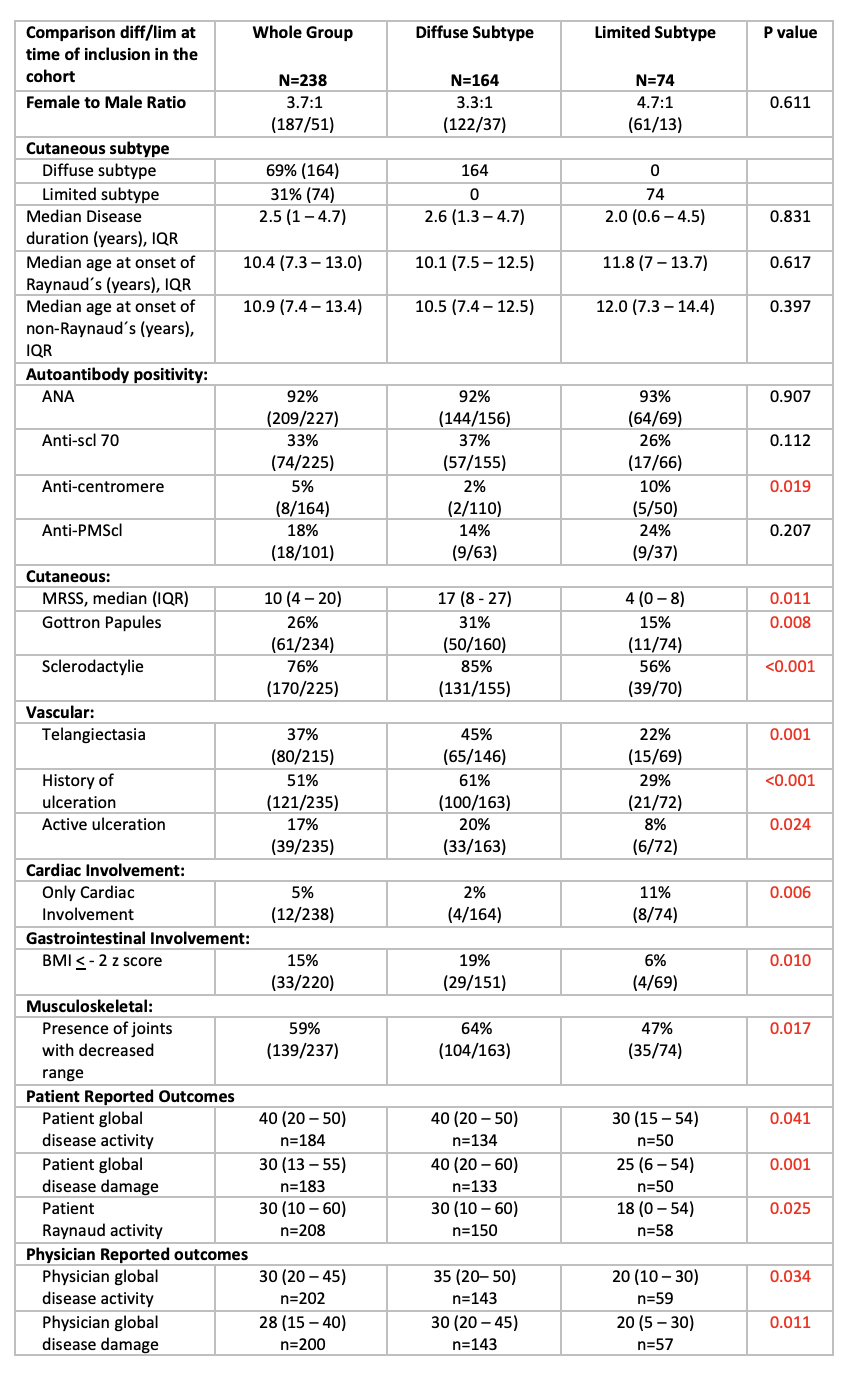
Results: The jSScC included 238 patients, 69% (n=164) had cutaneous diffuse subtype. The median age at onset of Raynaud’s phenomenon was 10.4 years (7.3-12.9) and the median age at the first non-Raynaud’s symptom was 10.9 years (7.3-13.0). Median disease duration was 2.5 years (1.0-4.7). The female/male ratio was not significantly lower in the djSSc subtype (3.3:1 versus 4.7:1, p=0.611). Antibody profile was similar, with the exception of a significantly higher number of anticentromere positive patients in the ljSSc (10% versus 2%, p=0.019). Patients with djSSc had significantly higher modified Rodnan Skin Score (17 versus 4, p=0.011), more frequently sclerodactyly (85% versus 56%, p< 0.001), Gottron papules (31% versus 15%, p=0.008), a history of digital ulceration (61% versus 29%, p< 0.001), active ulceration (20% versus 8%, p=0.024),telangiectasia (45% versus 22%, p=0.001), a decreased Body Mass Index (BMI) z score ≤ -2 (19% versus 6%, p=0.010) and decreased joint range of motion (64% versus 47%, p=0.017). Patients with ljSSc had significantly higher rate of cardiac involvement (11% versus 2%, p=0.006). There was no difference between the groups regarding sicca symptoms, pulmonary involvement assessed by FVC, DLCO and high resolution lung CT; renal involvement; gastrointestinal involvement beside decreased BMI < -2 z score; and muscle weakness.
Regarding patient related outcomes assessed by visual analogue scales (VAS0 to 100), djSSc patients had more severe disease related to patient reported global disease activity (40 versus 30, p=0.041), patient reported global disease damage (40 versus 25, p=0.001), and patient reported Raynaud activity by(30 versus 18, p=0.025). Additionally, physician related outcomes assessed by visual analogue scales (VAS), the physician reported global disease activity (35 versus 20, p=0.034), and physician reported global disease damage (30 versus 20, p=0.011), were significantly higher in djSSc patients.
Conclusion: In the largest jSSc cohort in the world, djSSc patients have significantly more severe disease according to patient and physician related outcomes than ljSSc patients. Patients with djSSc also had more cutaneous, vascular, and musculoskeletal involvements and patients with ljSSc had more cardiac involvement. Interestingly, we found no significant differences regarding interstitial lung disease, pulmonary hypertension or gastrointestinal involvement, although the number of patients with decreased BMI ≤-2 z score was significantly higher in the djSSc patients.
This project was supported by an unrestricted grant from “Joachim Herz Stiftung”
To cite this abstract in AMA style:
Foeldvari I, Klotsche J, Torok K, Kasapcopur O, Adrovic A, Feldman B, SZTAJNBOK F, TErreri M, Sakamoto A, Johnson S, Anton J, Stanevica V, Khubchandani R, Schonenberg-Meinema D, Al-Abadi E, Alexeeva E, Katsikas M, Sawhney S, Smith V, Appenzeller S, Avcin T, Kostik M, Lehman T, Malcova H, Marrani E, Pain C, Vasquez-Canizares N, Costa Reis P, Janarthanan M, Santos M, Abu Alsaoud S, Battagliotti C, Berntson L, bica b, Brunner J, Kaiser D, Lazarevic D, Minden K, Nuruzzaman F, Opsahl Hetlevik S, Uziel Y, Helmus N. Diffuse Juvenile Systemic Sclerosis Patients Show Distinct Organ Involvement, Antibody Pattern and Have More Severe Disease in the Largest jSSc Cohort of the World. Results from the Juvenile Scleroderma Inception Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/diffuse-juvenile-systemic-sclerosis-patients-show-distinct-organ-involvement-antibody-pattern-and-have-more-severe-disease-in-the-largest-jssc-cohort-of-the-world-results-from-the-juvenile-scleroder/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/diffuse-juvenile-systemic-sclerosis-patients-show-distinct-organ-involvement-antibody-pattern-and-have-more-severe-disease-in-the-largest-jssc-cohort-of-the-world-results-from-the-juvenile-scleroder/