Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The largest multi-center collaborative study on lymphoproliferative disorders (LPD) in rheumatoid arthritis (RA) (RA-LPD) in Japan was conducted to characterize its clinical outcomes and identify suitable treatments.
Methods: Patients with RA who developed LPD between January 1999 and March 2021 were retrospectively analyzed in a multicenter collaborative study across 48 hospitals in Japan. Significant differences were evaluated using Fisher’s exact test, the Mann-Whitney U-test, the Log-rank test, and a multivariate analysis with the Cox proportional hazard model. Significance was set at p < 0.05.
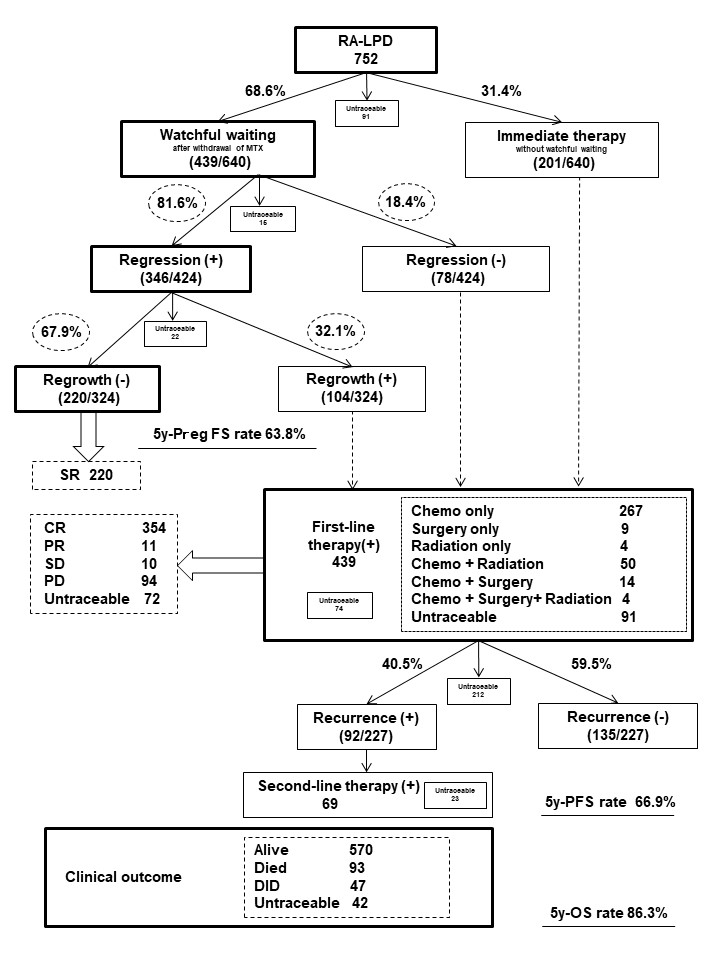
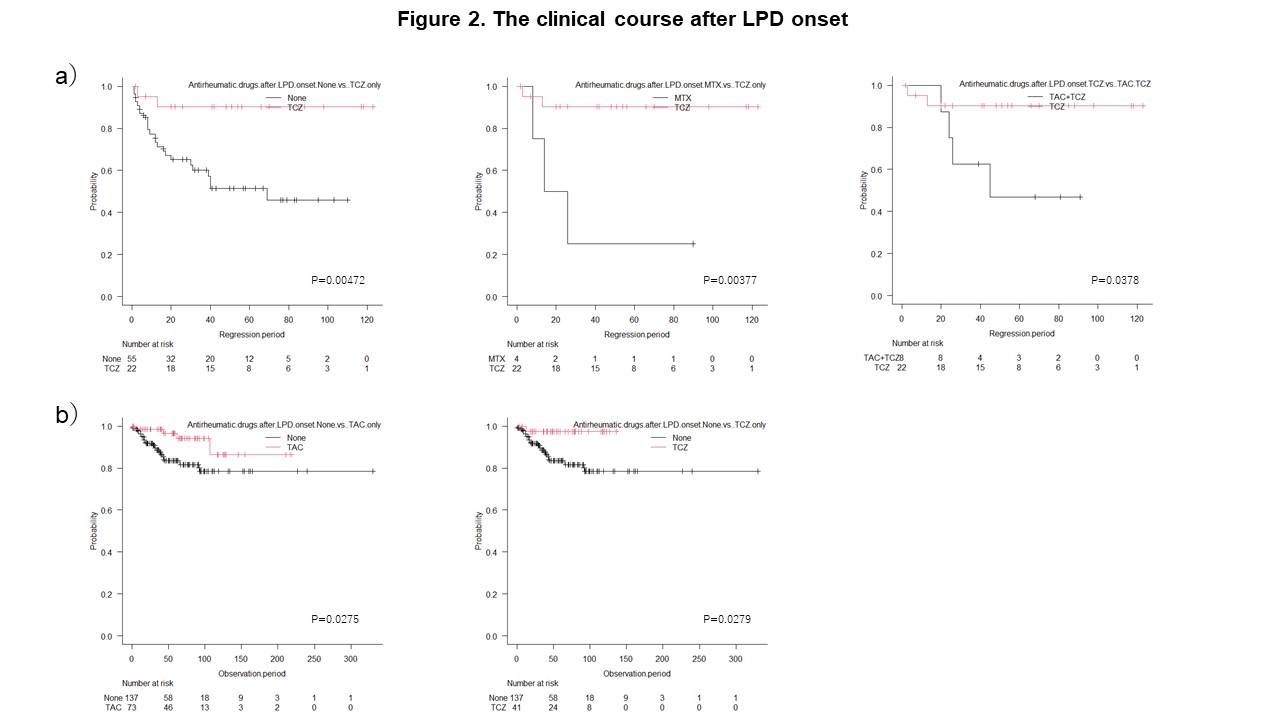
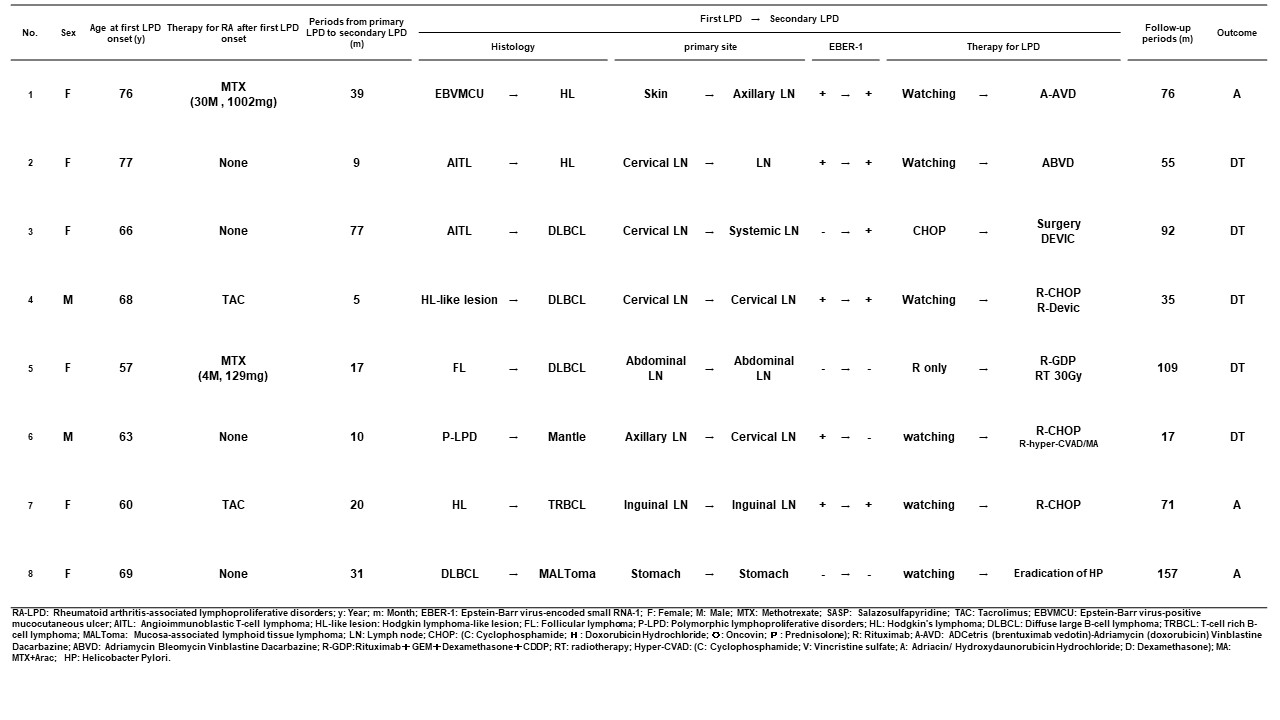
Results: Clinical outcomes of RA-LPD A total of 752 RA-LPD patients were enrolled. Their clinicopathological characteristics were presented at the ACR Convergence 2022. As shown in Figure 1, among 438 patients, 81.4% spontaneously regressed after the withdrawal of immunosuppressive agents, while 32.0% showed regrowth after a median of 12 months (range: 1-92). Among the 439 patients treated with first-line therapy, 365 (77.8%) achieved a complete or partial response to first-line therapy. The 5-year overall survival (OS) rate was 86.3%. The multivariate analysis identified an advanced clinical stage, Hodgkin lymphoma as independent regrowth after spontaneous regression, an older age ( >70 years), the T cell phenotype, and a sIL-2R level >1300 U/mL as independent unfavorable prognostic factors. Necessity for re-biopsy in patients with regrowth or relapse Eight patients with RA-LPD developed different histological subtypes after the regression or remission of RA-LPD. Among them, 6 patients (5.7%) showed regrowth after temporary tumor regression following the withdrawal of methotrexate (MTX), and 2 (2.2%) relapsed during temporary remission after chemotherapy. The tumor-related death rate was significantly higher in these patients (62.5%) than in those who developed LPD with the same histology (24.6%) (p=0.030) (Table 1). Therefore, re-biopsy is required for patients with regrowth or relapse. Recommended anti-rheumatic drug regimens after LPD onset The effects of antirheumatic drugs administered after the onset of LPD on the clinical outcomes of RA-LPD were examined in 393 patients with the relevant information available. The trastuzumab (TCZ) only group maintained a significantly higher rate of spontaneous regression than the none, MTX only, and TCZ plus tacrolimus (TAC) groups (Figure 2a). The prognosis of patients after the onset of LPD was better in the TAC only and TCZ only groups than in the none group, which included patients who had never been treated with MTX, TAC, TCZ, tumor necrosis factor inhibitors, abatacept, or a Janus-activating kinase inhibitor (Figure 2b).
Conclusion: The present study showed the clinical outcomes of RA-LPD and identified independent factors associated with post-spontaneous regression (SR)-free survival (PSRFS) and overall survival. Based on the results obtained, TCZ only regimens are recommended after the onset of LPD and re-biopsy is required for patients with regrowth or relapse.
Among 752 patients with RA-LPD, 68.5% spontaneously regressed after the withdrawal of immunosuppressive agents. Among them, 81.4% showed tumor regression, while 32.0% showed regrowth with a median of 12 months (range: 1-92).
Kaplan-Meier curves of RA-LPD according to antirheumatic drugs administrated after the onset of LPD.
a). PSRFS curve of RA-LPD. The rate of spontaneous regression was significantly higher in the TCZ only group than in the none, MTX only, and TCZ plus TAC groups.
b) OS curve of RA-LPD. The prognosis of patients after the onset of LPD was better in the TAC only and TCZ only groups than in the none group.
To cite this abstract in AMA style:
HOSHIDA Y, Tsujii A, OHSHIMA S, SAEKI Y, YAGITA M, MIYAMURA T, Katayama M, KAWASAKI T, HIRAMATSU Y, Oshima H, MURAYAMA T, HIGA S, KURAOKA K, HIRANO F, ICHIKAWA K, KUROSAWA M, SUZUKI H, CHIBA N, SUGIYAMA T, MINAMI Y, NIINO H, IHATA A, SAITO I, MITSUO A, MAEJIMA T, KAWASHIMA A, TSUTANI H, TAKAHI K, KASAI T, SHINNO Y, TACHIYAMA Y, TERAMOTO N, TAGUCHI K, NAITO S, YOSHIZAWA S, ITO M, SUENAGA Y, Mori S, NAGAKURA S, YOSHIKAWA N, NOMOTO M, UEDA A, NAGAOKA S, TSUURA Y, SETOGUCHI K, SUGII S, Abe A, SUGAYA T, SUGAHARA H, KOSETO M, KUNUGIZA Y, IIZUKA N, YOSHIHARA R, YABE H, FUJISAKI T, MORII E, TAKESHITA M, SATO M, SAITO K, Matsui K, TOMITA Y, FURUKAWA H, Tohma S. Rheumatoid Arthritis-associated Lymphoproliferative Disorders: A Multi-center Analysis of Clinical Outcomes and Evaluation of Anti-rheumatic Drugs After LPD Onset [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/rheumatoid-arthritis-associated-lymphoproliferative-disorders-a-multi-center-analysis-of-clinical-outcomes-and-evaluation-of-anti-rheumatic-drugs-after-lpd-onset/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatoid-arthritis-associated-lymphoproliferative-disorders-a-multi-center-analysis-of-clinical-outcomes-and-evaluation-of-anti-rheumatic-drugs-after-lpd-onset/