Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Adherence to urate-lowering therapy (ULT) reduces the incidence of debilitating gout flares. Providing a cue for a behavior, reinforcing the behavior with a reward, and then repeating this process is known as the cue-reward-repetition principle. Over time, cues become more important and rewards less because the activity becomes automatic (a habit). We conducted a randomized, adaptive pilot trial to test whether leveraging cues and rewards to establish medication taking as a habit improves adherence to ULT.
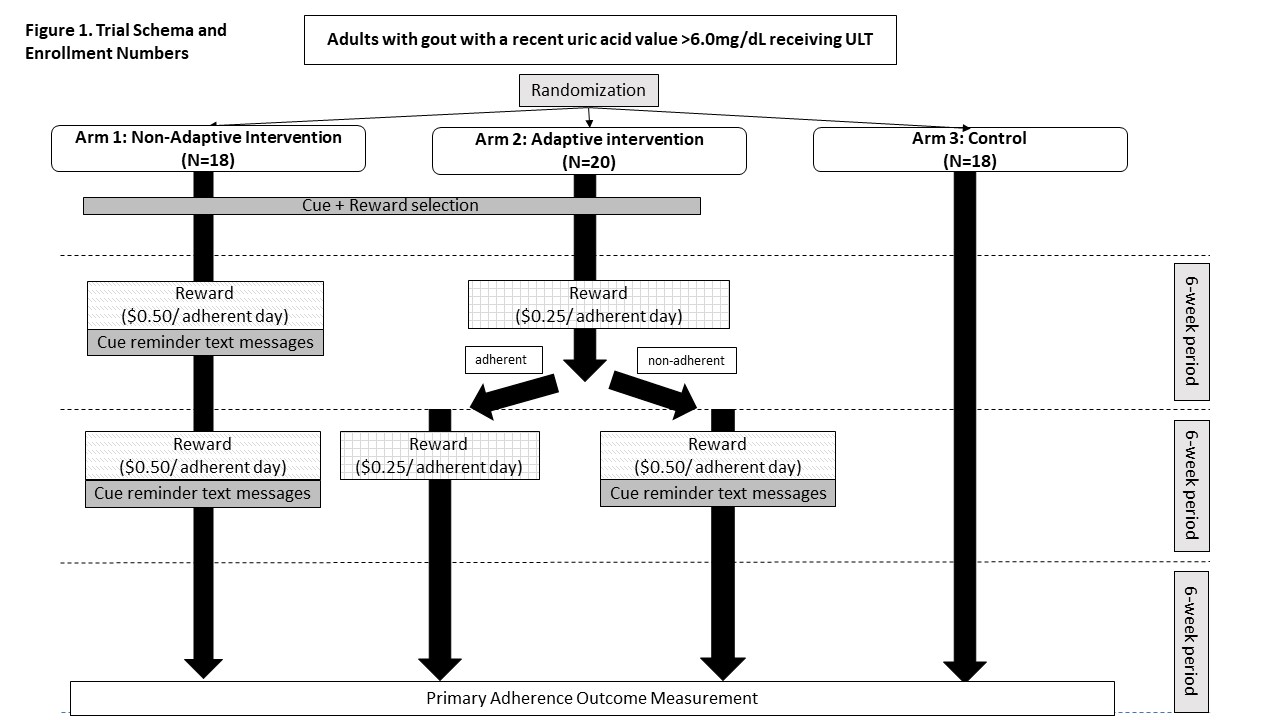
Methods: We enrolled adults 18 with gout and a uric acid level of >6mg/dL within 6 months, on a stable dose of ULT (allopurinol or febuxostat), who received rheumatology and/or primary care at a multisite medical center. Participants received electronic pill caps and were randomized to 3 arms: 1) the nonadaptive intervention where they received assistance selecting a cue (e.g., “place your medication near your toothbrush”) and a reward (charitable donation of $0.50/ULT adherent day) with text message reminders about their cue and the amount donated every 4 days, 2) the adaptive intervention with the same strategy but the reward started at $0.25/ULT adherent day and was doubled at 6 weeks if adherence was 75% 3) the control arm (pill cap monitoring only) (Fig. 1). The intervention ended at 12 weeks and adherence was monitored for the next 6 weeks. The primary outcome was adherence (number of times the pill cap was opened/number of doses prescribed per day) at 18 weeks. Secondary outcomes included adherence at 12 weeks, changes in automaticity, medication-taking routines and intentions, and uric acid level from baseline. We interviewed 14 patients who completed the trial to understand their experiences.
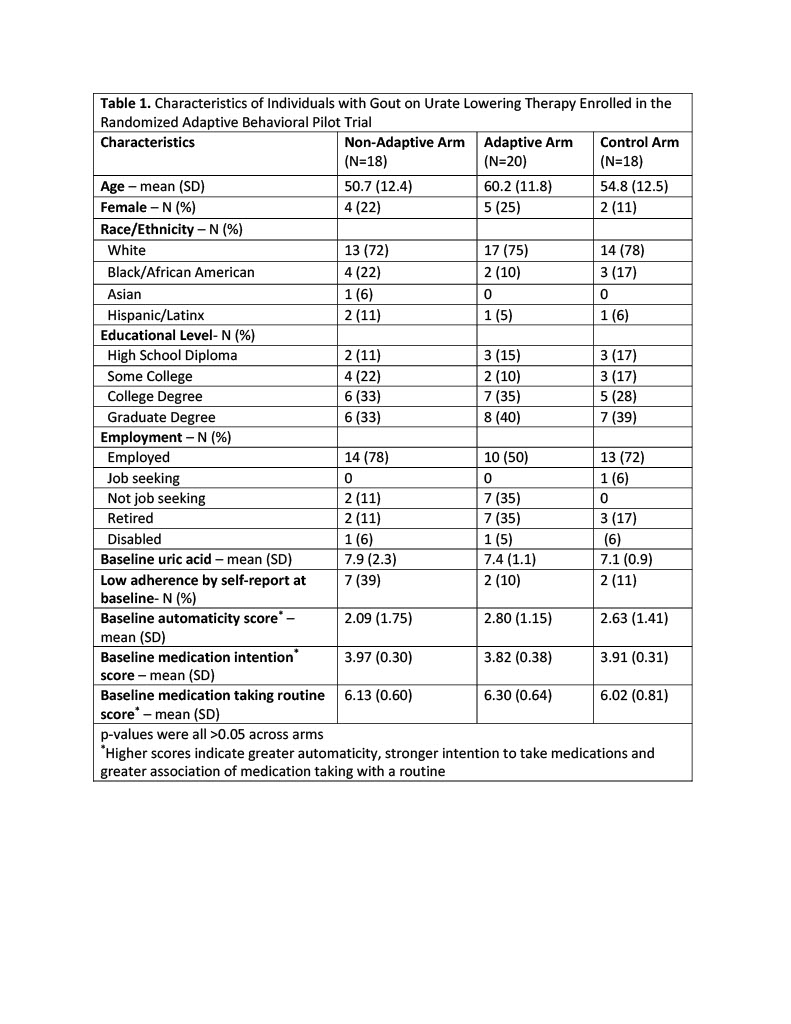
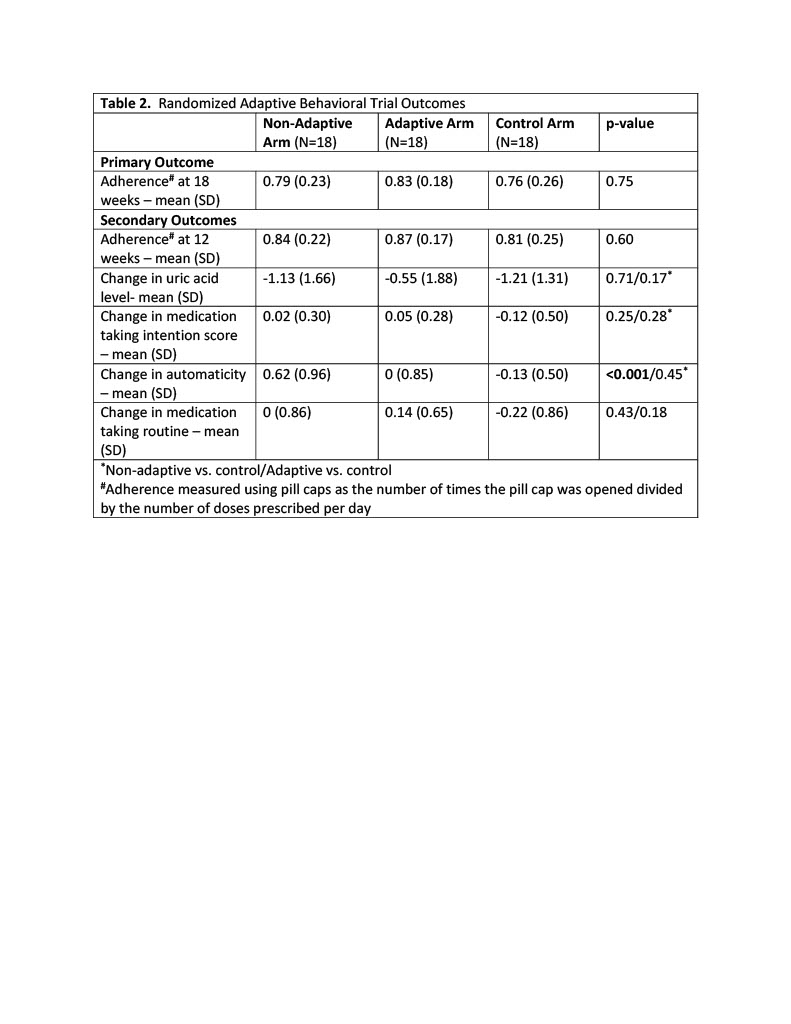
Results: Fifty-six individuals were randomized (non-adaptive arm (N=18), adaptive arm (N=20), and control arm (N=18)) (Fig. 1). Participants were 21% female, 81% White, 15% Black, 8% Latinx with similar demographics across arms (Table 1). The mean (SD) baseline uric acid was 7.47 (1.55) mg/dL. We did not observe statistically significant improvement in ULT adherence across intervention arms at 18 weeks (primary outcome) or at 12 weeks (secondary outcome) (Table 2). Mean (SD) adherence at 18 weeks was 0.79 (0.23) in the nonadaptive arm, 0.83 (0.18) in the adaptive arm, and 0.76 (0.26) in the control arm. Compared to the control arm, automaticity scores were higher post vs. pre intervention for the non-adaptive arm (p< 0.001) but not the adaptive arm (p=0.45); changes in other secondary outcomes were not significant. Exit interviews revealed limited intervention impact due to preestablished medication-taking habits, independent reasons to take ULT (e.g., prevention of painful gout flares) and ineffective reward (charity donation). Participants in all groups noted awareness of being monitored with the pill caps.
Conclusion: We did not observe significantly improved adherence to ULT among individuals enrolled in this habit formation intervention. High adherence in all arms and small sample size limited power. Larger studies over longer time frames are needed in diverse patient populations with varied baseline adherence to understand whether leveraging automaticity can enhance medication-taking behaviors.
To cite this abstract in AMA style:
Feldman C, Crum K, Hanken K, Fontanet C, Sears E, Oduol T, Vine S, Mastrorilli J, Bhatkhande G, Lauffenburger J, Oran R, Robertson T, Wood W, Choudhry N. Leveraging Cues and Rewards to Form Habits to Improve Medication Adherence in Gout: An Adaptive Behavioral Pilot Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/leveraging-cues-and-rewards-to-form-habits-to-improve-medication-adherence-in-gout-an-adaptive-behavioral-pilot-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/leveraging-cues-and-rewards-to-form-habits-to-improve-medication-adherence-in-gout-an-adaptive-behavioral-pilot-trial/