Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib (TOFA, NCT02592434) and Baricitinib (BARI, NCT03773978) are Janus kinase inhibitors (JAKi) that are approved for or being tested for nsJIA treatment. We aim to evaluate the effectiveness and safety profiles of JAKi, vs. biological agent (BA) with or without Methotrexate (MTX) combination in treating children with nsJIA.
Methods: Studies written in English language and published in ClinicalTrial.gov, PubMed, EMBASE, Cochrane are searched from establishment of the databases to May 2023. Randomized studies conducted in the nsJIA population, investigated effectiveness of JAKi or BA, and reported JIA-ACR responses with/without adverse event outcomes are eligible. The primary outcome of clinical efficacy was measured by JIA-ACR70 responses at 16+/-4 weeks and secondary outcomes include serious adverse events (SAE). Bayesian Network meta-analyses (BNMA) evaluate the effectiveness and safety outcomes of JAKis and BAs with/without MTX.BNMA are conducted separately for efficacy and safety outcomes. League table reports results for all pairwise comparisons.
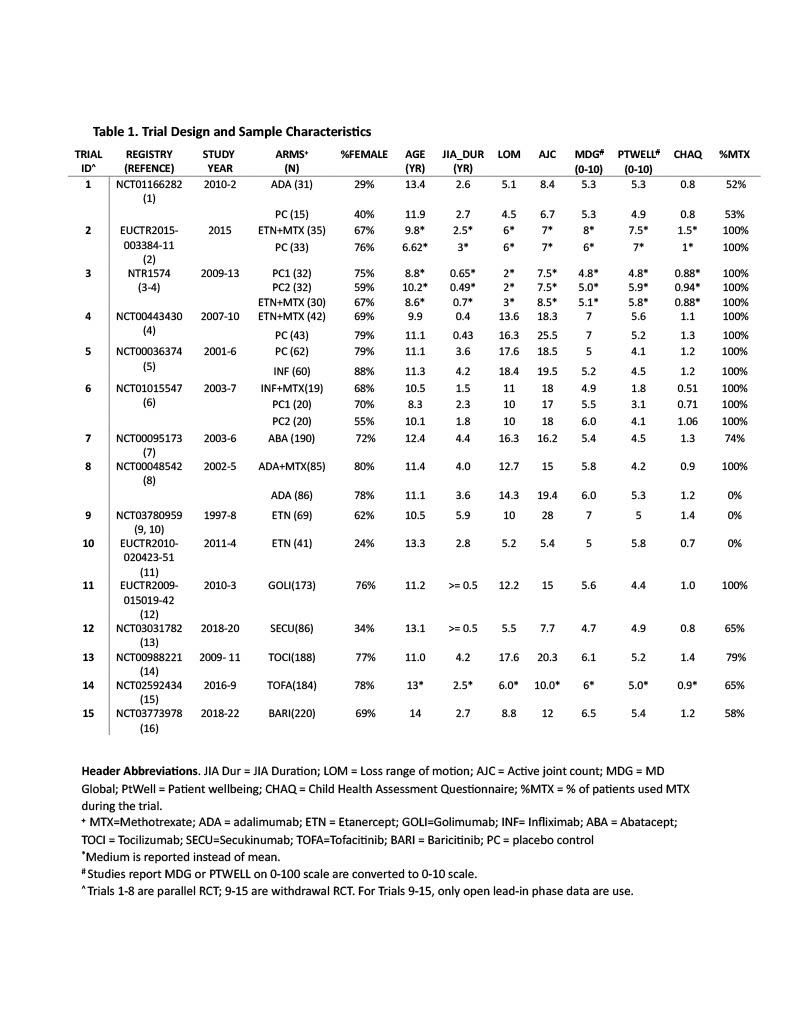
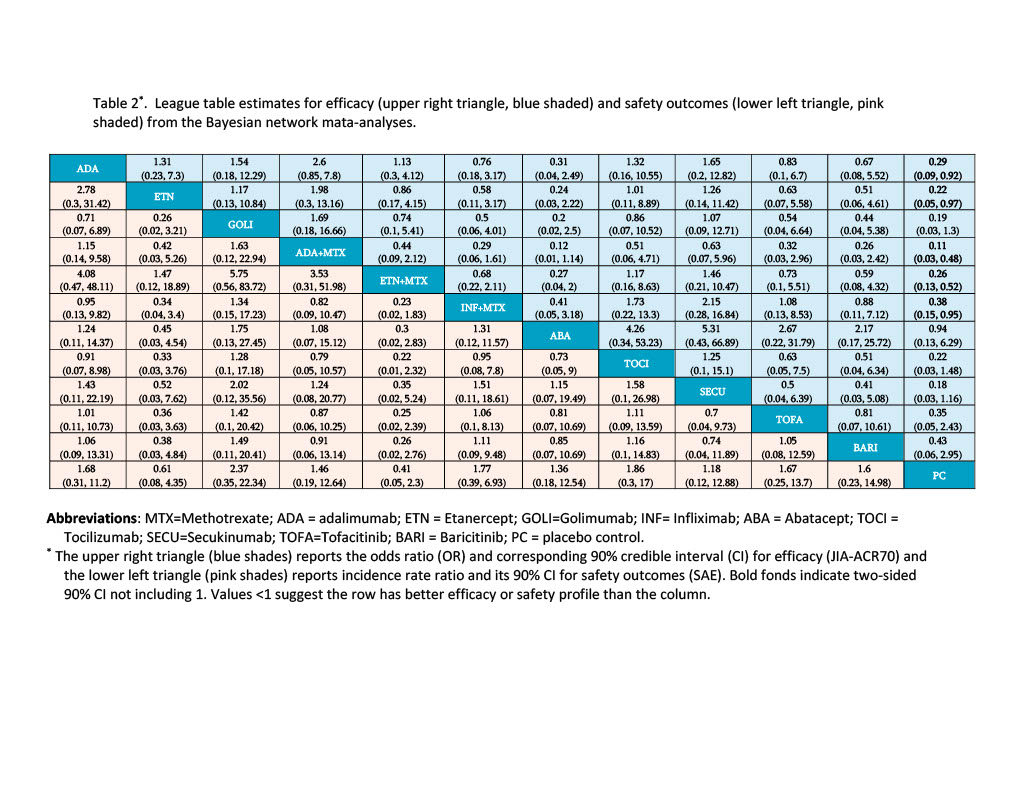
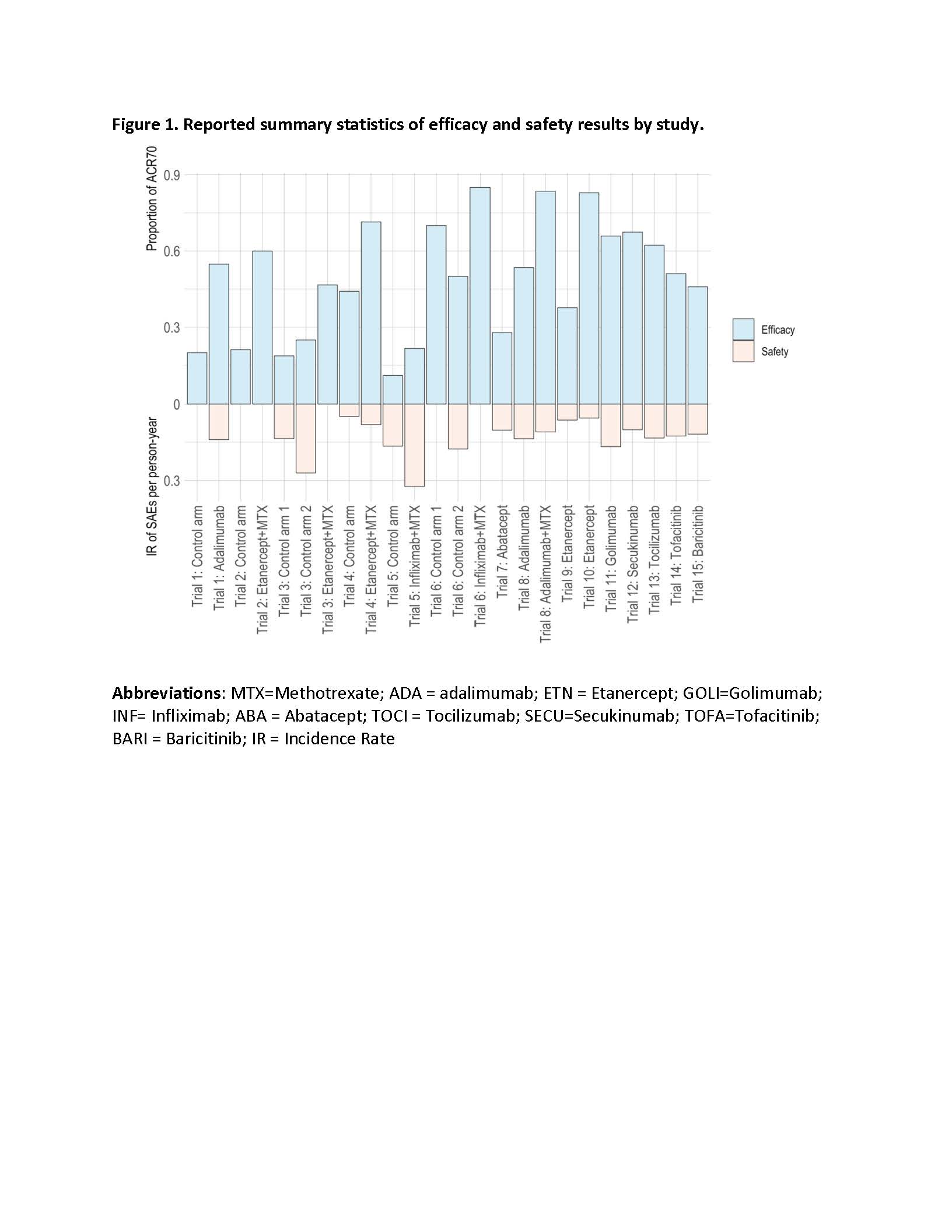
Results: The initial search query yielded 2,475 citations. Fifteen randomized controlled trials (RCT, 6 parallel and 9 withdrawal trials) met the study inclusion criteria. The meta-analyses investigated 6 BA, 3 BA+MTX combination, and two JAKi (i.e. TOFA and BARI). MTX background treatment was allowed, rarely mandated. The trial design and sample characteristics are summarized in Table 1. The aggregate summary statistics reported for the efficacy and safety outcomes are presents in Figure 1. The BNMA analyzed data from a total of 1,796 patients, estimates of odds ratio (OR) and corresponding 90% credible intervals are reported in Table 2 for the efficacy (upper triangle) and safety outcomes (lower triangle).
Conclusion: No significant differences are observed for pairwise comparisons among TOFA, BARI and BAs either alone or in combination with MTX for efficacy and safety outcomes when used for treating children with nsJIA. The BNMA indirect comparisons are limited, given that trials differ greatly in participants age, duration of disease, % of MTX background therapy, whether the trial mandate patients with inadequate responses to composition of DMARD or NSAID to be eligible, as well as the composition of JIA subtypes. The SAE may be defined differently in different studies. Future studies will expand the meta-analyses by including non RCT studies and individual patients’ data.
1. Burgos-Vargas R, Tse SML, Horneff G, et al. A Randomized, Double-Blind, Placebo-Controlled Multicenter Study of Adalimumab in Pediatric Patients With Enthesitis-Related Arthritis. Arthritis Care Res (Hoboken). 2015;67(11):1503_1512. doi:10.1002/acr.22657 ;
2. Alexeeva E, Horneff G, Dvoryakovskaya T, et al. Early combination therapy with etanercept and methotrexate in JIA patients shortens the time to reach an inactive disease state and remission: results of a double-blind placebo-controlled trial. Pediatr Rheumatol Online J. 2021;19(1):5. doi:10.1186/s12969-020-00488-9 ;
3. Hissink Muller PCE, Brinkman DMC, Schonenberg D, et al. A comparison of three treatment strategies in recent onset non-systemic Juvenile Idiopathic Arthritis: initial 3-months results of the BeSt for Kids-study. Pediatr Rheumatol Online J. 2017;15(1):11. doi:10.1186/s12969-017-0138_4 ;
4. Wallace CA, Giannini EH, Spalding SJ, et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum. 2012;64(6):2012_2021. doi:10.1002/art.34343 ;
5. Visvanathan S, Wagner C, Marini J, et al. The effect of infliximab plus methotrexate on the modulation of inflammatory disease markers in juvenile idiopathic arthritis: analyses from a randomized, placebo-controlled trial. Pediatric rheumatology. 2010;8:24-TN: NCT00036374/ClinicalTrials.gov. doi:10.1186/1546-0096-8_24 ;
6. Tynjala P, Vahasalo P, Tarkiainen M, et al. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (A-CUTE-JIA): A multicenter randomized clinical trial. Arthritis Rheum. 2009;60((Tynjala P.; Vahasalo P.; Tarkiainen M.; Aalto K.; Kroger L.; Malin M.; Putto-Laurila A.) Helsinki University, Central Hospital, Helsinki, Finland):2051. doi:10.1002/art.27123 ;
7. Ruperto N, Lovell DJ, Quartier P, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372(9636):383_391. doi:10.1016/S0140-6736(08)60998-8 ;
8. Lovell DJ, Ruperto N, Goodman S, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359(8):810-820. doi:10.1056/NEJMoa0706290 ;
9. Lovell DJ, Giannini EH, Reiff A, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342(11):763-769. doi:10.1056/NEJM200003163421103 ;
10. NCT03780959. Safety and Efficacy of Etanercept (Recombinant Human Tumor Necrosis Factor Receptor Fusion Protein [TNFR: fc]) in Children With Juvenile Rheumatoid Arthritis (JRA). https://clinicaltrials.gov/show/NCT03780959. Published online 2018. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01918680/full ;
11. Horneff G, Foeldvari I, Minden K, et al. Efficacy and safety of etanercept in patients with the enthesitis-related arthritis category of juvenile idiopathic arthritis: results from a phase III randomized, double-blind study. Arthritis Rheumatol. 2015;67(8):2240_2249. doi:10.1002/art.39145 ;
12. Brunner HI, Ruperto N, Tzaribachev N, et al. Subcutaneous golimumab for children with active polyarticular-course juvenile idiopathic arthritis: results of a multicentre, double-blind, randomised-withdrawal trial. Ann Rheum Dis. 2018;77(1):21_29. doi:10.1136/annrheumdis_2016_210456 ;
13. Brunner HI, Foeldvari I, Alexeeva E, et al. Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: a randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial. Ann Rheum Dis. Published online August 12, 2022:annrheumdis_2022_222849. doi:10.1136/ard_2022_222849 ;
14. Brunner HI, Ruperto N, Zuber Z, et al. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis. 2015;74(6):1110_1117. doi:10.1136/annrheumdis_2014_205351 ;
15. Ruperto N, Brunner H, Synoverska O, et al. Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet (london, england). 2021;398(10315):1984_1996. doi:10.1016/S0140-6736(21)01255_1 ;
16. Ramanan AV, Quartier P, Okamoto N, et al. Baricitinib in juvenile idiopathic arthritis: an international multi-centre phase 3, randomized, double-blind, placebo-controlled, withdrawal, efficacy, and safety trial. Lancet, In press.
To cite this abstract in AMA style:
Huang B, Li Y, Yue X, Andorf S, Lovell D, Brunner H. Network Meta Analyses of the Effectiveness and Safety Profiles of Janus Kinase Inhibitors and Biologic Agents in Treating Children with Non-systemic Juvenile Idiopathic Arthritis (nsJIA) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/network-meta-analyses-of-the-effectiveness-and-safety-profiles-of-janus-kinase-inhibitors-and-biologic-agents-in-treating-children-with-non-systemic-juvenile-idiopathic-arthritis-nsjia/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/network-meta-analyses-of-the-effectiveness-and-safety-profiles-of-janus-kinase-inhibitors-and-biologic-agents-in-treating-children-with-non-systemic-juvenile-idiopathic-arthritis-nsjia/