Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: Patient Outcomes, Preferences, & Attitudes I: Assessment Tools
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Despite taking strong immunosuppressive medications to control inflammation, many patients with rheumatoid arthritis (RA) continue to experience moderate to severe pain that does not correlate with laboratory or clinical findings. Sleep disturbances are extensively reported among patients with RA and could influence pain perception. Prior investigations have demonstrated that one night of partial sleep loss is associated with increases in next day pain in patients with RA. However, there is a knowledge gap regarding the impact of sleep disturbances on long-term pain outcomes. The objective of the present study was to determine whether sleep disturbances early in the disease are associated with future pain interference.
Methods: Data were from adults with early RA (joint symptoms ≤12 months) enrolled in the Canadian Early Arthritis Cohort between 2016-2023 who completed PROMIS (Patient-Reported Outcomes Measurement Information System) measures at 0, 6-, 12-, 18-, and 24-months to assess sleep disturbance (primary predictor) and pain interference (primary outcome). PROMIS sleep disturbance assesses perceptions of sleep quality, depth, and restoration. PROMIS pain interference measures the extent to which pain interferes with physical, mental, and social functioning. To estimate longitudinal associations between sleep disturbance and pain interference, the dataset was lagged so that repeat measures of sleep disturbance at 0, 6, 12 and 18 months were specified to predicted pain interference 6-months later at 6-,12-, 18- and 24-months follow up. Linear mixed effects regression was used to estimate crude and adjusted effects of sleep disturbance on pain interference over the 24-month study period adjusted for age, sex, body mass index (BMI), education, income, smoking status, comorbidity index, steroid use, and treatment.
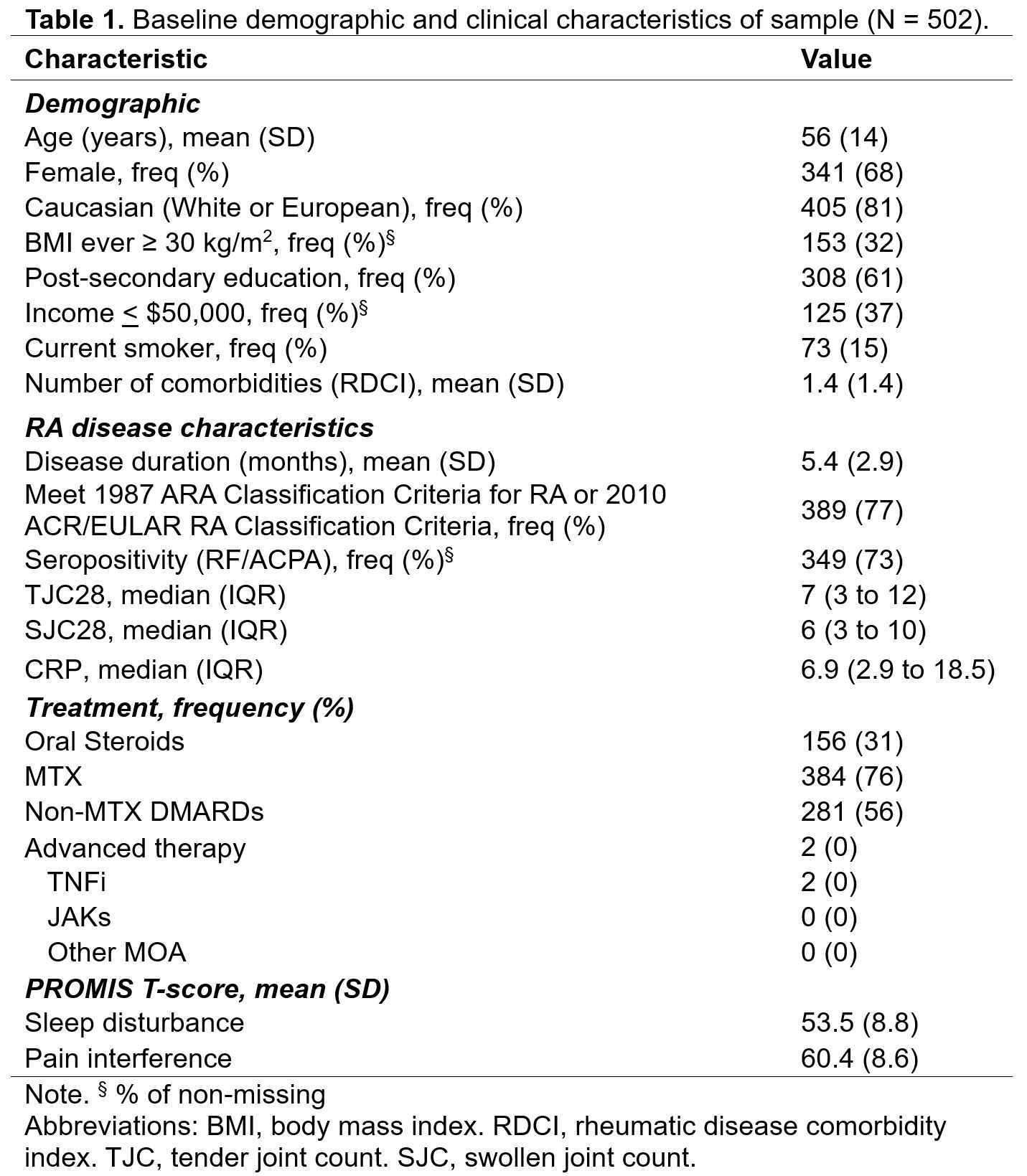
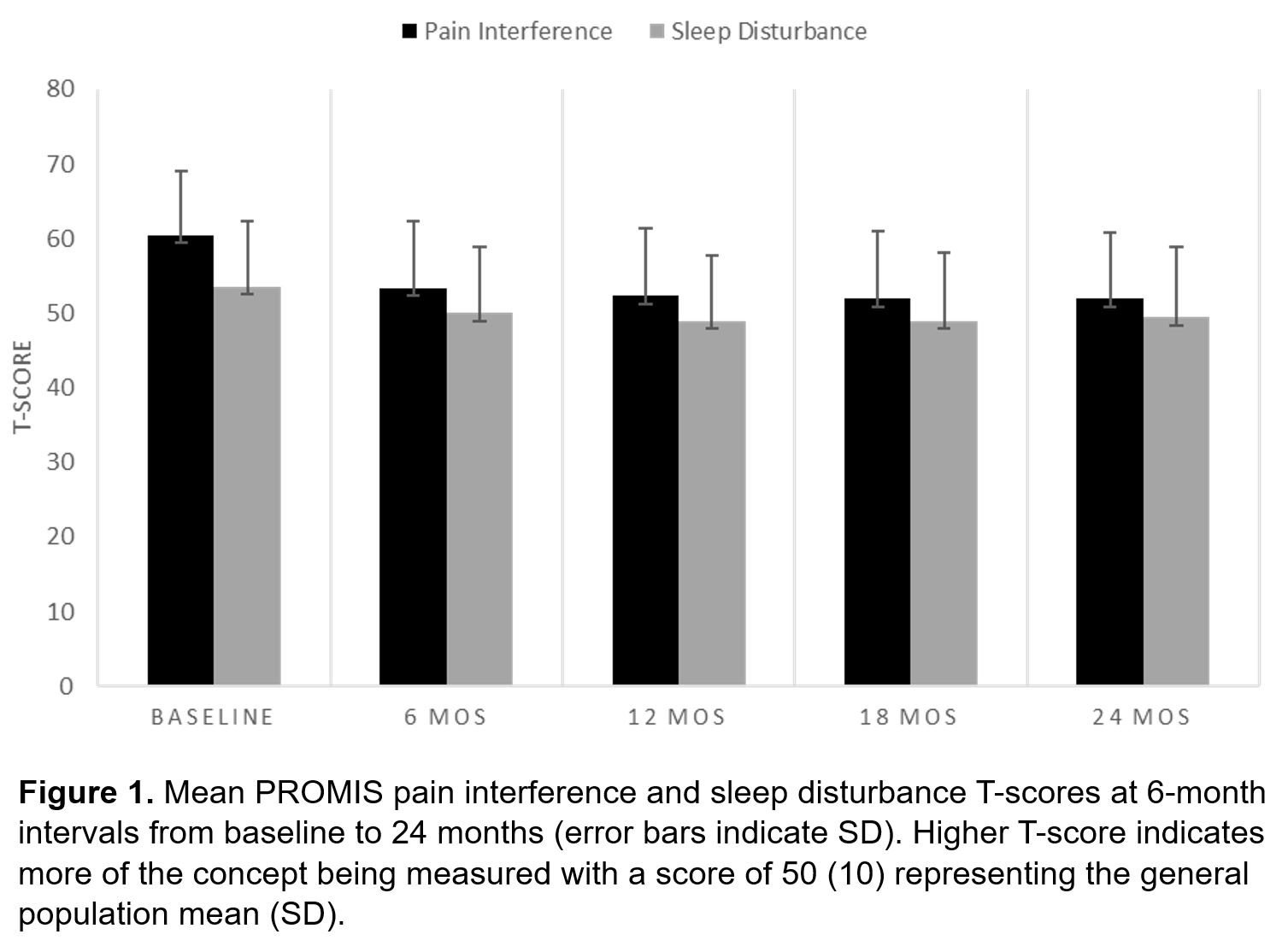
Results: The sample included 502 patients with early RA. At baseline, the sample was 68% female, 81% Caucasian, 73% seropositive (RF/ACPA), and 77% met the 1987 ARA Classification Criteria for RA or the 2010 ACR/EULAR RA Classification Criteria; with a mean (SD) age of 56 (14) years, BMI of 28.4 (6.7) kg/m2,and disease duration of 5.4 (2.9) months (Table 1). The mean (SD) T-score for PROMIS pain interference was 60.4 (8.6) and PROMIS sleep disturbance was 53.5 (8.5) at baseline (Figure 1). The unadjusted and adjusted linear mixed effects regression models revealed a significant association between sleep disturbance and pain interference scores, indicating that better sleep 6-months prior was associated with less pain interference at the following 6-month evaluation (Table 2).
Conclusion: In this cohort of patients with early RA, more disturbed sleep predicted greater pain interference 6 months later. These findings underscore the importance of addressing sleep disturbances as part of pain management strategies. Identifying and targeting problematic sleep disturbances early on may help improve long-term pain outcomes. Further work is needed to explore the underlying mechanisms and to investigate potential interventions targeting sleep to alleviate pain in RA.
To cite this abstract in AMA style:
Aydemir B, Schieir O, Valois M, Muhammad L, Song J, Dunlop d, Bartlett S, Bessette L, Boire G, Hazelwood G, Hitchon C, Keystone E, Pope J, Thorne C, Tin D, Bykerk V, Lee Y, (CATCH) Investigators C. Sleep Disturbance Predicts Pain Interference in Patients with Early Rheumatoid Arthritis in a Prospective Real-World Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/sleep-disturbance-predicts-pain-interference-in-patients-with-early-rheumatoid-arthritis-in-a-prospective-real-world-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sleep-disturbance-predicts-pain-interference-in-patients-with-early-rheumatoid-arthritis-in-a-prospective-real-world-cohort/