Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: Metabolic & Crystal Arthropathies – Basic & Clinical Science
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: To optimally manage gout, the ACR recommends a treat-to-target (T2T) strategy, which entailsthe titration of urate-lowering therapy (ULT) to achieve and maintain a serum urate (SU) level of <6.0 mg/dL.Current data indicates that T2T using conventional ULT may be required for up to 2 years to reduce gout flare frequency compared to usual care (Doherty et al, Lancet 2018). Using data from the STOP Gout Study (O’Dell JR et al. NEJM Evidence 2022), a recently completed multicenter, randomized, double-blind, non-inferiority trial, we examined whether achievement of SU goalat 48 weeks reduces gout flare burdenas early as 48-72 weeks after T2T initiation.
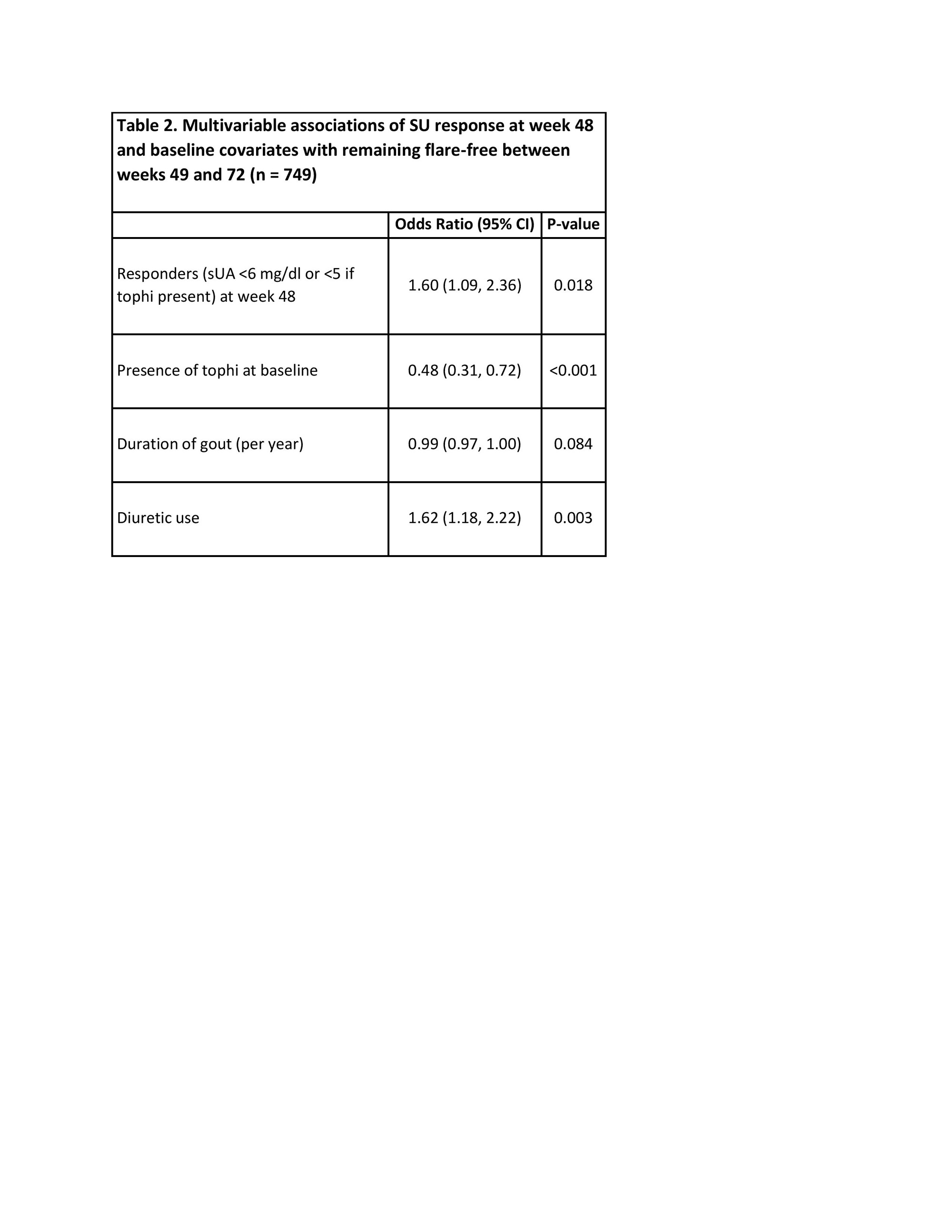
Methods: Trial participants with gout and SU concentration ≥6.8 mg/dL were randomized 1:1 to receive allopurinol or febuxostat. ULT was titrated during weeks 0-24 (Phase 1) and maintained during weeks 25-48 (Phase 2), with escalation as necessary to reach goal SU of <6.0 mg/dl (<5 mg/dl if tophi) or until maximum dosing achieved. Participants were then observed on stable ULT with a primary study outcome of at least 1 flare occurring during weeks 49-72 (Phase 3).SU goal achievement was assessed at 48 weeks. For this analysis, the association of SU goal achievement at the end of Phase 2 with flare during Phase 3 was assessed in a multivariable logistic regression model, adjusting for covariates significantly associated with flare in univariate analysis. Covariates assessed included baseline age, sex, race, education, comorbidities, body mass index (BMI), quality of life (EQ5D score), and gout-related factors (baseline SU, tophi, gout duration, diuretic use, prior allopurinol use).
Results: Of the 940 trial participants (mean age 62 years, 98% male, mean [SD] baseline SU 8.5 [1.4] mg/dl), 749 had flare data available during Phase 3 and were included in this analysis. The most common reasons for early termination were either participant decision (n=99) or loss to follow-up (n=41). Of the 749 with flare data, 449 (60%) remained flare-free between week 49 and week 72. Patients achieving SU goal at 48 weeks were more likely than those not achieving SU goal to remain flare-free during Phase 3 (62% vs. 49%; p = 0.004 by chi-square). In unadjusted analyses, other baseline factors associated with flare during Phase 3 included longer duration of gout (p = 0.009) and presence of tophi (p-value < 0.001), while baseline diuretic use was associated with a lower risk (p = 0.004) (Table 1). After adjusting for tophi, disease duration, and diuretic use, SU goal achievement at 48 weeks was associated with remaining flare-free in Phase 3(OR 1.60; 95% CI 1.09-2.36, p = 0.018; Table 2).
Conclusion: In this post-hoc analysis from a large, randomized double-blind, non-inferiority trial, we found that SU achievement following a T2T strategy was associated with a 60% greater odds of remaining flare-free between 49 and 72 weeks of follow-up. These results support ACR guidelines that endorse a T2T strategy in gout management and demonstrate that benefits in flare prevention may begin to occur as early as 12 to 18 months.
To cite this abstract in AMA style:
Qu J, Helget L, Androsenko M, Wu H, Kramer B, Newcomb J, Brophy M, Davis-Karim A, England B, Ferguson R, Pillinger M, Neogi T, Palevsky P, O'Dell J, Mikuls T. Treat-to-target Urate-lowering Therapy Reduces Gout Flare Burden: Post-hoc Analysis of a Multicenter, Randomized, Double-blind, Non-inferiority Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/treat-to-target-urate-lowering-therapy-reduces-gout-flare-burden-post-hoc-analysis-of-a-multicenter-randomized-double-blind-non-inferiority-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treat-to-target-urate-lowering-therapy-reduces-gout-flare-burden-post-hoc-analysis-of-a-multicenter-randomized-double-blind-non-inferiority-trial/