Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: New guidelines published in 2020 conditionally recommend HLA-B*58:01 allele testing for South Asian and Black patients receiving allopurinol to reduce the risk of severe cutaneous adverse events. However, no data exists on population-wide genetic testing rates. We investigated HLA-B*58:01 testing among allopurinol users in the Veterans Health Administration (VHA) Healthcare System.
Methods: Using data from the VHA Corporate Data Warehouse (CDW), we identified facilities with structured fields available for capturing HLA-B*58:01 test results. From these facilities, we identified all patients with a current, active prescription for allopurinol as of December 2022. We assessed the proportion of patients with a documented HLA-B*58:01 test at any time within the CDW, by race or ethnicity. We repeated this analysis among incident users of allopurinol (defined as users with a prescription in 2022 and no allopurinol use in the preceding 3 years). Finally, we examined variation in HLA-B*58:01 testing among self-identified Asian or Black patients by VHA facility, among facilities with ≥ 20 eligible patients. We contacted the top performing facility to learn about local workflows for obtaining genetic testing for allopurinol users.
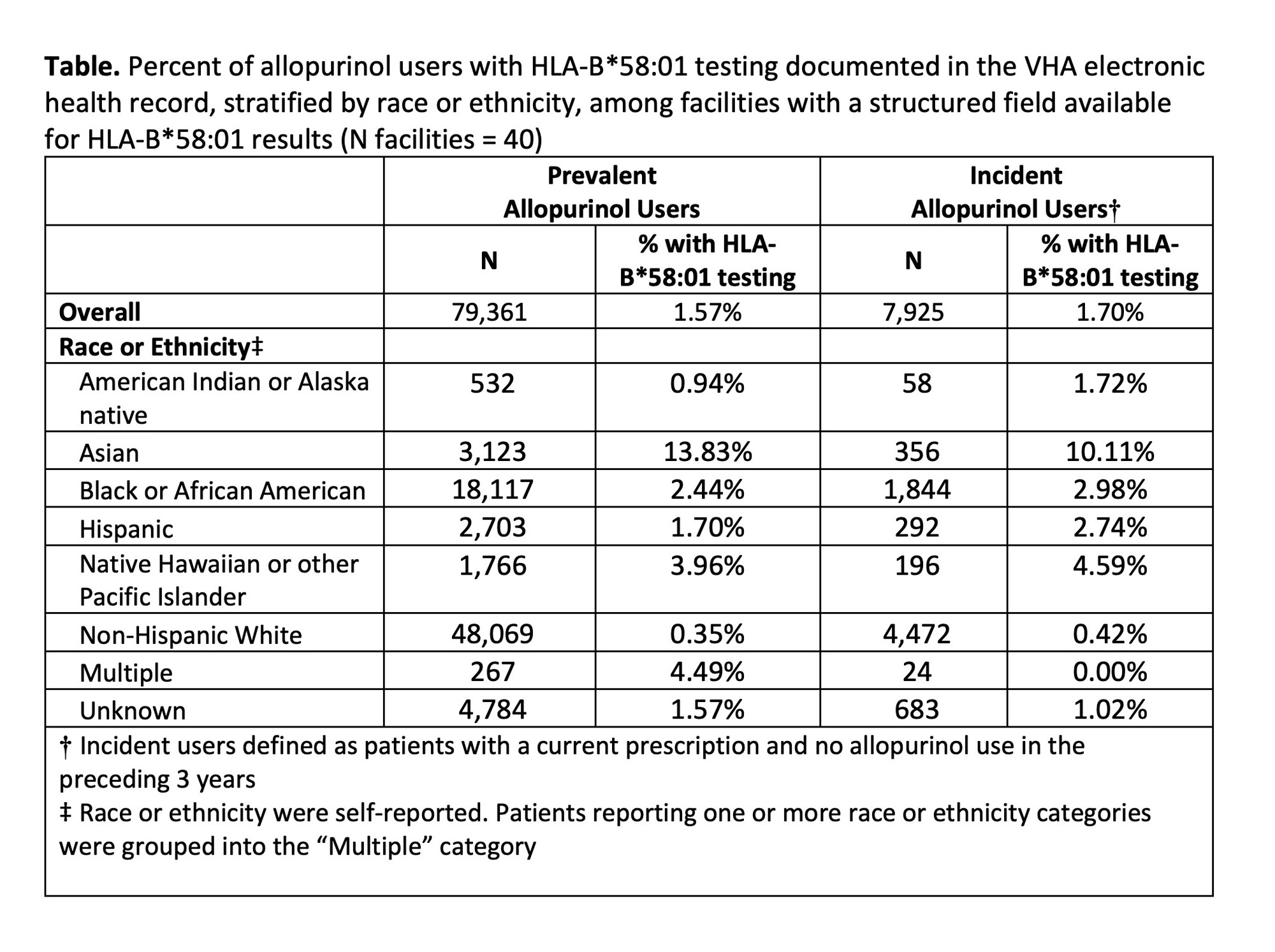
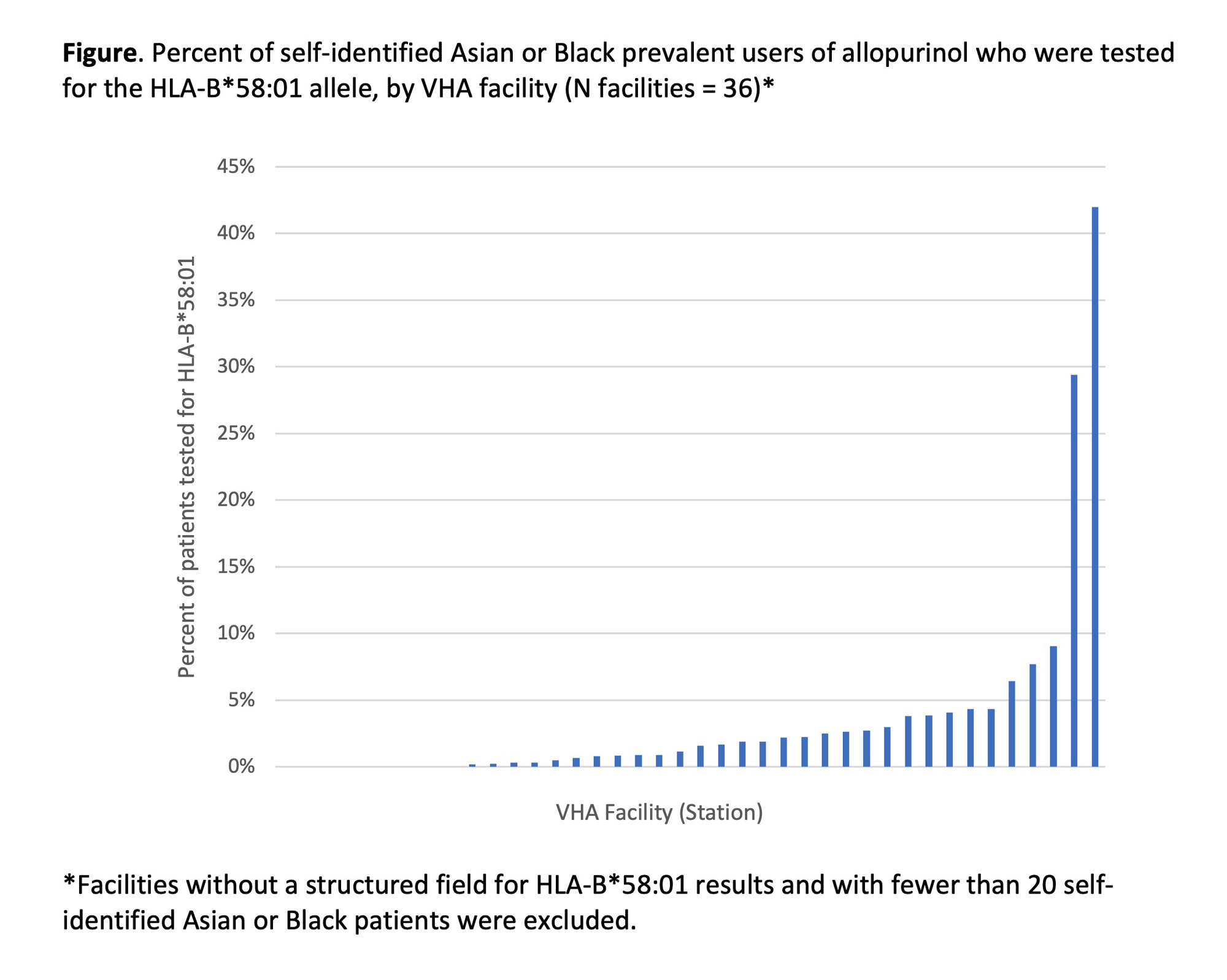
Results: Only 40 out of 130 facilities had a structured field available for capturing HLA-B*58:01 test results. We identified 79,361 users of allopurinol at these facilities; 98% of them were male, and mean age was 70 years (SD 12). Among prevalent users, 13.8% of Asian and 2.4% of Black patients were ever tested for the HLA-B*58:01 allele (Table). Among incident users, testing was documented among 10.1% of Asian and 3.0% of Black patients. We observed wide variation in HLA-B*58:01 testing across facilities (range 0% – 42.0%; Figure). The top-performing facility (proportion tested 42.0%) relayed that they had developed an allopurinol order set and pharmacy workflow that prevented allopurinol prescribing prior to completion of genetic testing.
Conclusion: Across the VHA Healthcare System, testing for HLA-B*58:01 in high-risk groups was very low, and variation across facilities was high. Most facilities lack a structured field for HLA-B*58:01 results, which hinders measurement of quality gaps. In order to improve testing rates, facilities should consider implementing order sets or pharmacy “hard stops” that require HLA-B*58:01 testing for high-risk groups.
To cite this abstract in AMA style:
Sullivan J, Ware A, Tarasovsky G, Wilson C, Whooley M, Singh J, Yazdany J, Schmajuk G. Facility Variation in HLA-B*58:01 Allele Testing for Asian and Black Patients Receiving Allopurinol in the Veterans Affairs Healthcare System [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/facility-variation-in-hla-b5801-allele-testing-for-asian-and-black-patients-receiving-allopurinol-in-the-veterans-affairs-healthcare-system/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/facility-variation-in-hla-b5801-allele-testing-for-asian-and-black-patients-receiving-allopurinol-in-the-veterans-affairs-healthcare-system/