Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Effective and expeditious control of rheumatoid arthritis (RA) disease activity using a treat-to-target (T2T) strategy is crucial to prevent long term damage and disability. T2T often uses a trial-and-error approach in selecting therapeutics and assessing clinical response after 3-6 months. If patients have not reached a state of low disease activity or remission, the cycle of trial and error is often repeated until satisfactory disease control is achieved, which can result in long delays in getting to a low disease state. Imaging with Tc99m tilmanocept (TIL), a high affinity ligand to CD206 expressed on activated macrophages, may provide an early predictor of clinical response, generating an objective, quantifiable readout of changes in macrophage density in joint inflammation of patients undergoing initiation or change of bDMARD therapy. Changes in macrophage density may be observed within weeks of treatment initiation, long before many clinical readouts used in standardized disease assessments become apparent.
Methods: 30 RA patients from a Phase 2b trial (NCT03938636) with active RA (DAS28 ≥ 3.2; ACR/EULAR 2010 Classification Criteria ≥ 6) set to start anti-TNFα therapy were enrolled and followed for 24 weeks. Hand/wrist planar gamma camera images were obtained one hour post IV administration of TIL at baseline prior to initiation of new treatment, as well as at 5, 12, & 24-weeks post-therapy initiation (N= 28/30 subjects; 2 lost to follow up). Images were quantitatively assessed to detect localization within synovial spaces of bilateral hands and wrists by determining average pixel intensity in each region of interest relative to average pixel intensity in an adjacent reference region, followed by comparison to a normative database of healthy control subject images. A panel of established clinical assessments (ACR 20/50/70, CDAI, DAS28, HAQ-DI) was performed at each time point to compare imaging results with clinical evaluations.
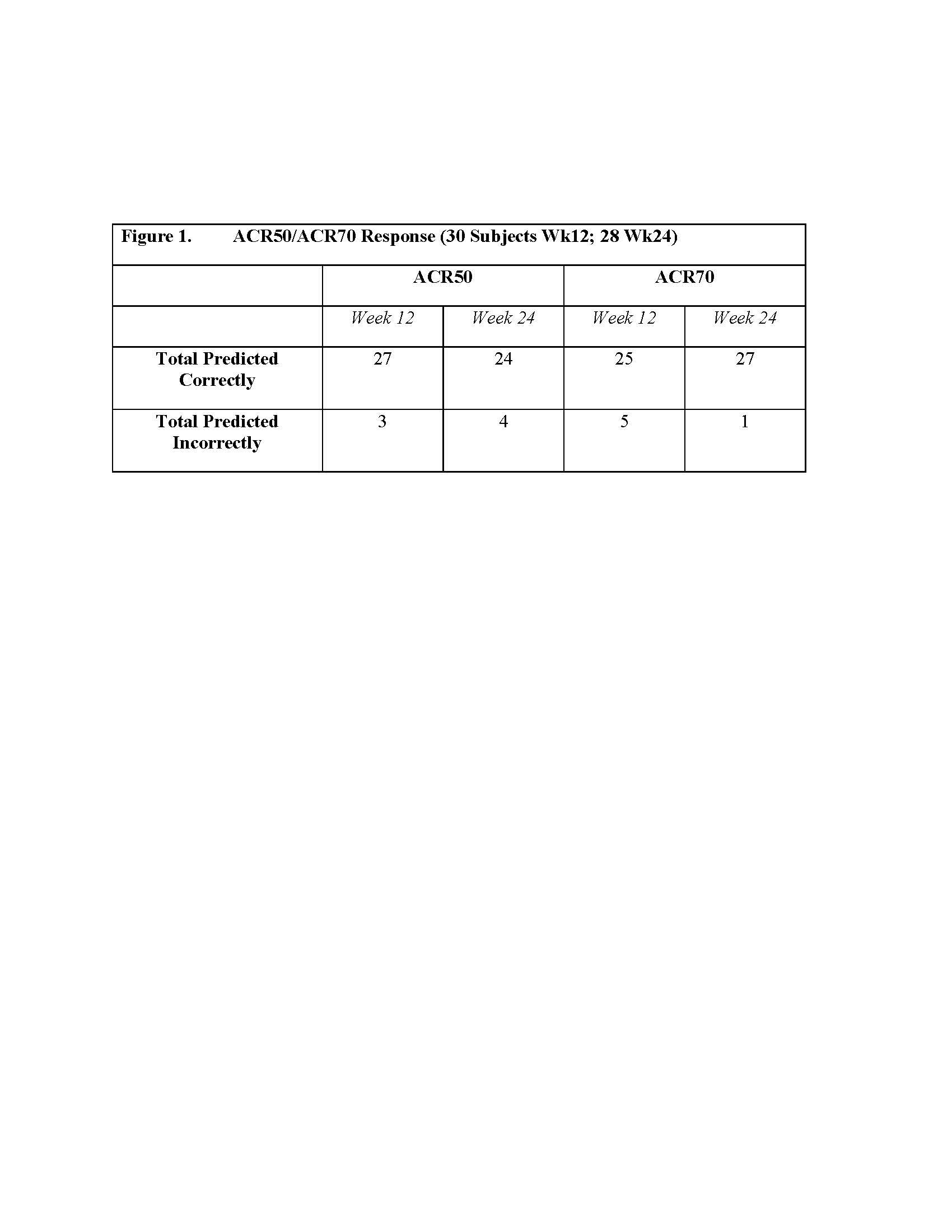
Results: In 27/30 subjects, TIL imaging from baseline to week 5 predicted ACR50 response at 12 weeks (Fig 1). For 24/28 subjects, TIL imaging from baseline to week 5 was predictive of ACR50 response at 24 weeks (Fig 1). There were 5 ACR50 responders at both weeks 12 and 24. Using ACR70 response as representation of clinical outcome, 25/30 subjects were correctly predicted at 12 weeks and 27/28 at 24 weeks. (Fig 1). Truth tables were generated comparing ACR50 clinical response or non-response at weeks 12 and 24, demonstrating high specificity (0.96 at weeks 12 and 24), PPV, and NPV (week 12: PPV=0.75, NPV=0.92; week 24: PPV=0.67, NPV=0.88). Sensitivity values (week 12= 0.6; week 24= 0.4) were possibly influenced by the low number of ACR50 responders in this cohort (N=5). Combining TIL quantitative image analysis with serological and clinical markers in a multivariate model gave an AUC of 0.95 for prediction of treatment response at week 24.
Conclusion: These results indicate that marked changes in TIL global uptake values by week 5 presage clinical efficacy evaluations at week 12 and week 24 of treatment and demonstrate that tilmanocept imaging can provide quantifiable imaging assessment of RA-involved joints that enables an objective, early prediction of clinical response.
To cite this abstract in AMA style:
Graf J, Jacobs M, Gladd-Foley D, Ruiz-Irizarry Y, Kivitz A, Churchill M, Kardan A, Leach M, Ralph D, Korczak N, Hasselbach A, Abbruzzese B, Hershey R, Potter B, Fitzpatrick J, Thornton A, Blue M, Rosol M. Tc99m Tilmanocept Imaging Predicts Clinical Response in Rheumatoid Arthritis Patients Beginning New Anti-TNFα Therapy [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/tc99m-tilmanocept-imaging-predicts-clinical-response-in-rheumatoid-arthritis-patients-beginning-new-anti-tnf%ce%b1-therapy/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tc99m-tilmanocept-imaging-predicts-clinical-response-in-rheumatoid-arthritis-patients-beginning-new-anti-tnf%ce%b1-therapy/