Session Information
Date: Monday, November 14, 2022
Title: T Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: In rheumatoid arthritis (RA), T cells represent a large proportion of the immune population present in inflamed joint synovium, and subsets of both CD4+ and CD8+ T cells are suspected to contribute to the pathology of the disease. Here, we use single cell RNA-sequencing (scRNA-seq) to better understand T cell receptor (TCR) repertoire differences and relationships both across cellular subsets and between tissue compartments in RA.
Methods: We performed 5′ droplet-based scRNA-seq on lymphocytes sorted from synovial tissue biopsies (n = 12) and matched peripheral blood samples (n = 10) to obtain paired transcriptomic and repertoire information for captured cells. After filtering low-quality cells and performing an initial round of clustering, T cells were identified, populations of CD4+, CD8+, and innate T cells were separately re-clustered, and a combination of differentially-expressed gene analysis, gene-set enrichment, and reference mapping was utilized to clearly define the cell subpopulations present. Paired TCR information for each cell was then incorporated, allowing for metrics of clonal overlap, diversity, and expansion to be calculated among the subpopulations.
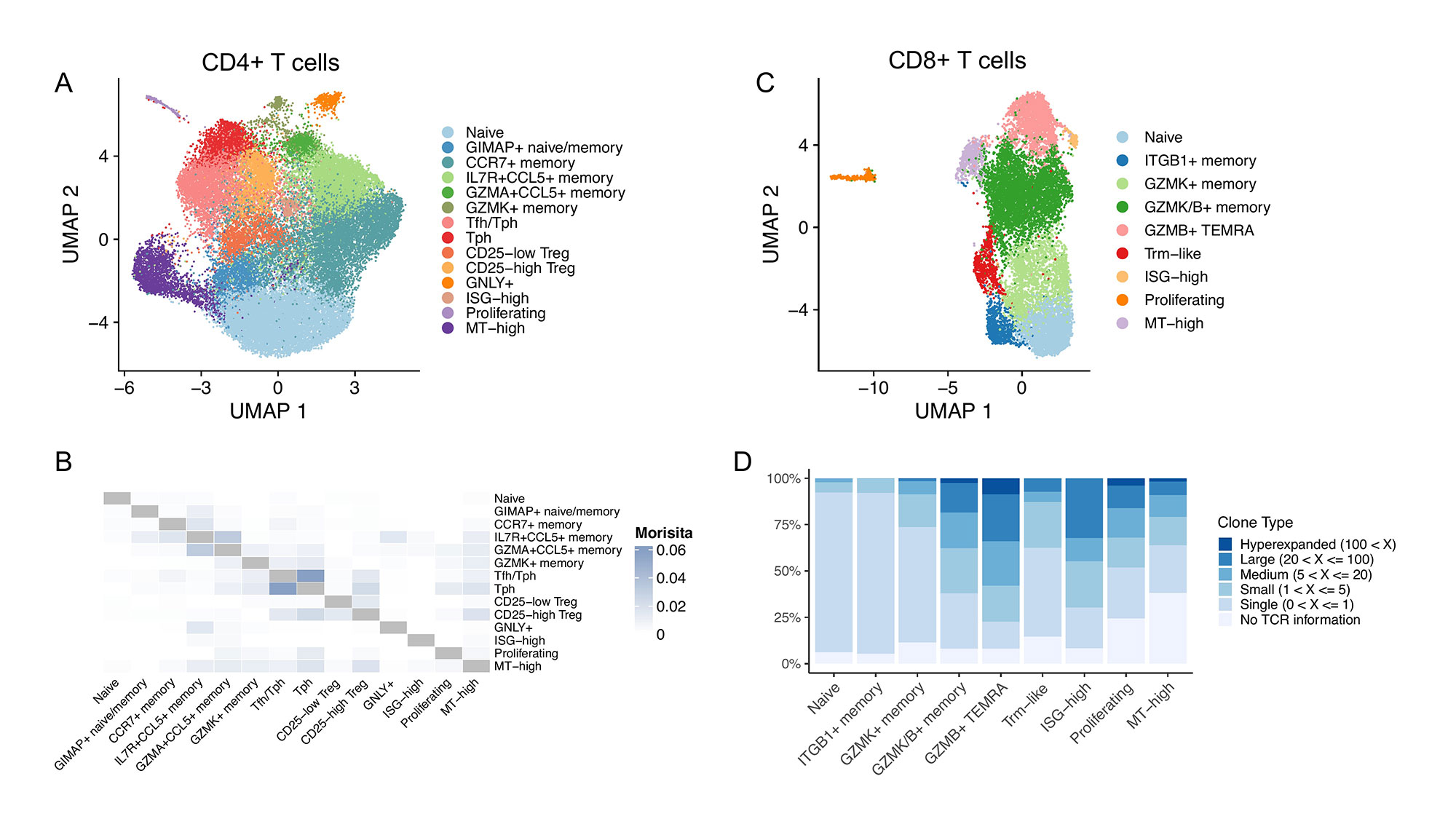
Results: Among 14 CD4+ clusters, we identified populations of naïve, memory, regulatory, T peripheral helper (Tph), T follicular helper (Tfh), cytotoxic, and proliferating T cells (Fig 1A). Naïve CD4+ cells had increased representation in the blood compared to the synovial tissue, while Tph, Tfh, and CD25high Treg cells were among the clusters significantly increased in synovial tissue. When analyzing the repertoire of these cells, we found the largest clonal expansion among the cytotoxic, Tph, and granzyme A (GZMA+) memory populations. An examination of shared clones between clusters found the Tph cluster to be clonally related to the Tfh cluster and to comprise a significant proportion of proliferating CD4+ cells in the synovial tissue (Fig 1B). A detailed comparison of clonal and non-clonal Tph cells revealed that expanded clones had elevated markers of effector function, including TNFRSF18, IFNG, and PRF1. Analysis of CD8+ T cells revealed 9 clusters, including populations of naïve, memory, cytotoxic, and proliferating cells (Fig 1C). We identified a population of cells expressing GZMK and GZMB, along with a TEMRA population, to be highly clonally expanded (Fig 1D). We then further characterized a potentially viral-reactive set of these CD8 clones by referencing a public database of viral TCRs. Finally, a focus on innate T cells confirmed the presence of a MAIT population by TCR, of which we could identify expanded clones shared between tissue and blood in multiple patients.
Conclusion: T cells are crucial for the development and progression of RA but features of the TCR repertoire in relation to the disease have not been adequately studied. Here, we provide a comprehensive look at the TCR repertoire of RA patients, both within synovial tissue and in the blood. We identify unique clonal attributes related to each of these compartments and identify clonal relationships among transcriptomically different populations of cells, which together deepen our understanding of the pathogenic role of certain T cell subsets in RA.
To cite this abstract in AMA style:
Dunlap G, Wagner A, Meednu N, Jonsson A, Zhang F, Wei K, Utz P, Robinson W, Maecker H, James J, Guthridge J, Bridges, Jr. S, Donlin L, Goodman S, DiCarlo E, Bykerk V, Ritchlin C, Tabechian D, Lederer J, Gravallese E, McGeachy M, Firestein G, Gregersen P, Horowitz D, Boyle D, Geraldino-Pardilla L, Perlman H, Mandelin A, Bathon J, Hughes L, Holers V, Deane K, Moreland L, Filer A, Pitzalis C, Forbess L, Ben-artzi A, Salomon-Escoto K, Raychaudhuri S, Brenner M, RA/SLE Network A, McDavid A, Anolik J, Rao D. Single-Cell Characterization of the TCR Repertoire Across Tissue and Blood in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/single-cell-characterization-of-the-tcr-repertoire-across-tissue-and-blood-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-cell-characterization-of-the-tcr-repertoire-across-tissue-and-blood-in-rheumatoid-arthritis/