Session Information
Date: Monday, November 14, 2022
Title: T Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: T cell tolerance is essential for preventing autoimmune diseases and resolving inflammation. To maintain tolerance, CD4+ T cells recognizing self-antigens in the periphery can exert active suppression as T regulatory (Treg) cells or else enter an unresponsive state known as anergy. Recent evidence suggests that anergy occurs naturally within a subpopulation of self-reactive polyclonal CD4+ T cells and can generate precursors for Foxp3+ Treg cell differentiation.
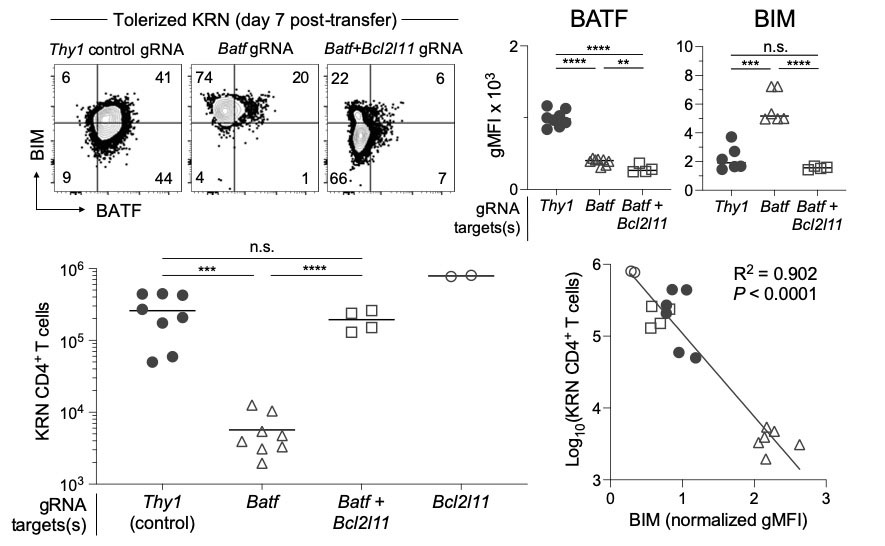
Methods: Polyclonal CD4+ T cell subsets from C57BL/6 mice were physically sorted for the purpose of downstream sequencing-based interrogation. Anergic CD44high Foxp3– CD73high FR4high Nrp1+ T cells were compared to effector/memory (CD44high Foxp3– CD73low FR4low), Treg (Foxp3+ CD25+), and naïve (CD44low) T cell populations. Divided aliquots from the same samples were processed in parallel to generate corresponding accessible chromatin DNA (ATAC-seq) and RNA-seq information. Bioinformatic analyses were applied to identify unique transcription factor (TF) networks in the anergic T cell subpopulation. Anergy-enriched TFs were then investigated in antigen-specific tolerization systems with germline knockout mice and CRISPR-Cas9 gene editing of primary T cells.
Results: Integrated ATAC-seq and RNA-seq analysis revealed a novel TF program in anergic T cells driven by the activator protein 1 (AP-1) family member BATF (Batf). Anergy signature gene targets uniquely demonstrated open-conformation chromatin with enriched BATF consensus binding motifs and foot-printing analyses confirmed TF binding to AP-1–interferon regulatory factor (IRF) composite elements (AICEs). Remarkably, tolerized Batf-deficient T cells demonstrated reduced PD-1 (Pdcd1) expression and defective cell cycle arrest – consistent with failed anergy – and were eventually lost to clonal deletion. Mechanistically, this new deletional tolerance was driven by increased expression of the pro-apoptotic protein BIM (Bcl2l11) in the absence of BATF. Simultaneous knockdown of Batf and Bcl2l11 restored anergic T cell survival during tolerization. Finally, Batf-deficient T cells failed to give rise to a stable population of anergy-derived Foxp3+ Treg cells.
Conclusion: Anergic T cells display a unique gene-regulatory TF program driven by a BATF-containing AP-1 protein complex interacting with an IRF protein at AICE elements. Batf gene knockdown experiments suggest that BATF-dependent transactivation at checkpoint inhibitor genes such as Pdcd1 leads to the proliferative arrest of anergic cells. Furthermore, BATF upregulation during anergy induction is essential to suppress the expression of BIM and ensure the survival of anergic T cells and anergy-derived Foxp3+ Tregs.
To cite this abstract in AMA style:
Titcombe P, Silva-Morales M, Zhang N, Mueller D. BATF Represses BIM Expression to Sustain the T Cell Anergy Program [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/batf-represses-bim-expression-to-sustain-the-t-cell-anergy-program/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/batf-represses-bim-expression-to-sustain-the-t-cell-anergy-program/