Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: In September 2021, the FDA updated its boxed warnings for Janus Kinase (JAK) inhibitors to include increased risk of major adverse cardiac events (MACE) and cancer in addition to preexisting warnings about thrombosis. These new potential risks need to be taken into context with the benefits of medication as well as individual patient risk factors. Currently, we do not have a systematic approach to communicating with patients about new risks. This quality improvement (QI) project aimed to improve rates and approach of communicating these risks of JAK inhibitors to patients with rheumatic diseases at the Dallas VA.
Methods: We identified consecutive patients on JAK inhibitors returning for rheumatology follow-up between 11/2021-5/2022. We randomized (computer generated) each patient to one of two existing approaches of communicating risk (of MACE, cancer, or DVT): a) using a gist (summary) explanation of FDA warnings or b) presenting visual aids (icon arrays shown on poster) of risk per outcome. We reviewed the medical record/rheumatology notes to compare documentation rates of risk communication for JAK inhibitors any time prior to initiation of this QI project (reflecting the 2019 FDA thrombosis warnings). Upon completion of either communication strategy, we administered a survey to evaluate perceived confidence with decision-making using the 4-item SURE Decisional Conflict scale.
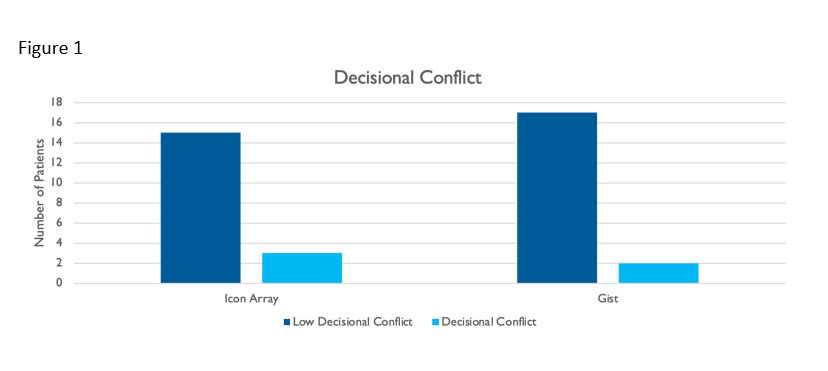
Results: We included 37 Veterans; 80% were male with an average age of 63. Over 75% of the patients had rheumatoid arthritis, 15% had psoriatic arthritis, and 8% had ankylosing spondylitis. Nearly all patients (89%) had one or more cardiovascular risk factors. Baseline documentation rates for risk communication were 11 of 36 (31%) reflecting 2019 FDA warnings, and increased to 37/37 (100%) with this project. Five (14%) of the patients in both groups changed to alternative therapy following communication of risks: two due to potential risks, one due to history of a pertinent risk factor, and two due to lack of JAK inhibitor efficacy. A similar number of patients changed therapy in each communication approach; 2 of 19 in the gist and 3 of 18 in the icon array group for varying reasons. Post-intervention survey results revealed 17 of 19 patients (89%) in the gist and 15 of 18 patients (83%) in the icon array approaches reported low decisional conflict according to the SURE scale (figure 1).
Conclusion: In this ongoing QI project, we have increased rates of communicating risk and learned that either communication approach is well-received. Decisions are nuanced and rely on prior medication use, disease activity, and risk for adverse outcomes. As long-term safety data are released, we must learn to effectively communicate and document risks with patients using shared decision-making.
To cite this abstract in AMA style:
Weisberg R, Capel B, Guo A, Patel K, Arora R, Reddy S, Makris U. Quality Improvement: Communicating Risk Regarding JAK Inhibitor Use in Rheumatology Patients [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/quality-improvement-communicating-risk-regarding-jak-inhibitor-use-in-rheumatology-patients/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quality-improvement-communicating-risk-regarding-jak-inhibitor-use-in-rheumatology-patients/