Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Patients with rheumatic and musculoskeletal diseases (RMDs) require a tailored follow-up that is limited by the capacity of healthcare professionals. The implementation of innovative tools such as digital solutions in clinical practice may contribute to optimal manage patients with RMDs. We developed a study to test the feasibility and acceptance of Adhera Precision Digital Companion Platform™ for real-time monitoring of electronic patient reported outcomes (ePROs) in patients with rheumatoid arthritis (RA) and spondyloarthritis (SpA).
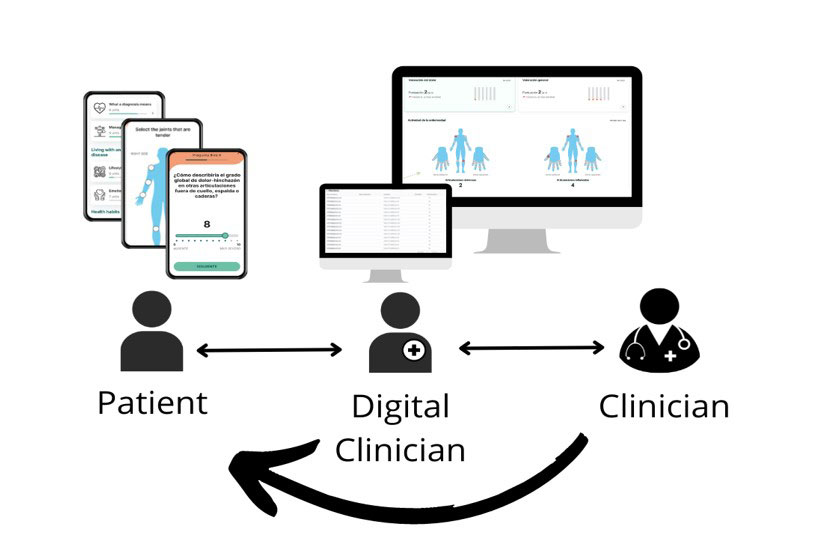
Methods: Digireuma was a 6-month prospective study including patients with RA and SpA, using the digital Precision Digital Companion Platform, Adhera® for Rheumatology. During follow-up, patients were asked to report disease specific ePROs on a pre-established basis in the mobile solution. In addition, flares, changes in medication and recent infections were available to be reported at any time. Two rheumatologists monitored these outcomes, and patients were contacted for online or face-to-face interventions when deemed necessary by clinicians (Figure 1). Assessment measures included patient global assessment (PGA) of disease activity, self-reported tender joint count (sr-TJC), self-reported swollen joint count (sr-SJC), Health Assessment Questionnaire (HAQ) and pain visual analogue scale (VAS) for patients with RA; PGA, sr-TJC, sr-SJC, BASDAI, and ASAS-HI for patients with SpA. All measures were delivered every two weeks, avoiding two questionnaires on the same day. Usability of the digital solution was measured by the Net-Promoter Score (NPS) and through qualitative telephone interviews.
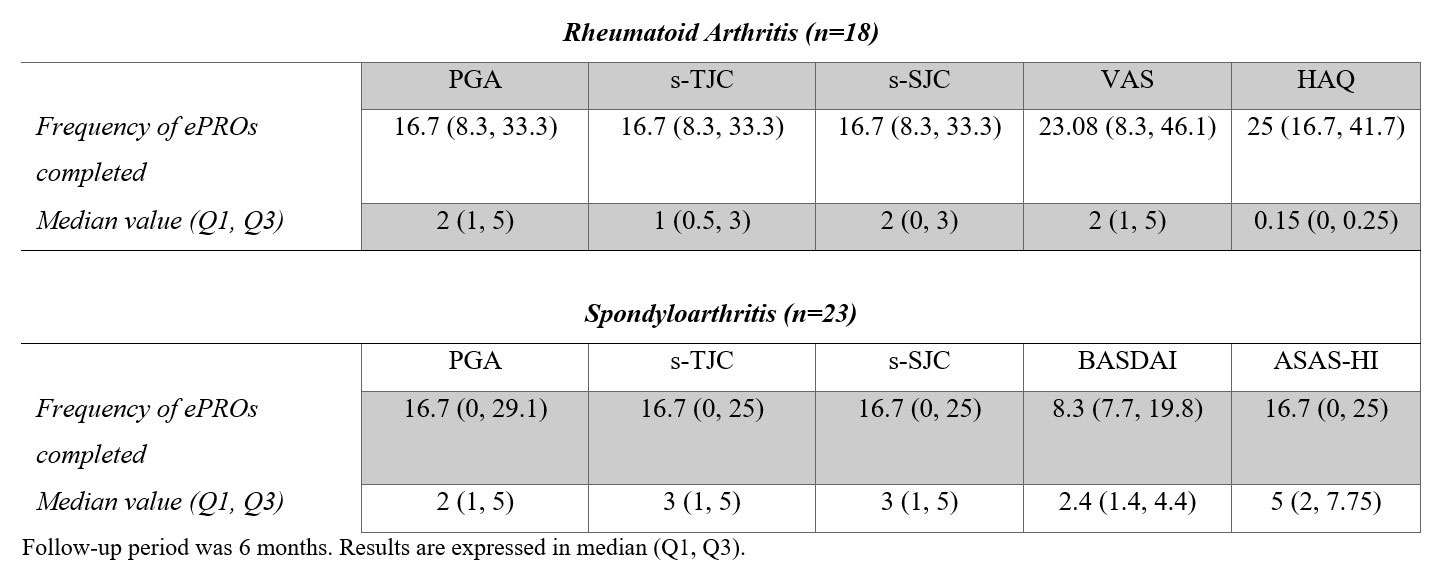
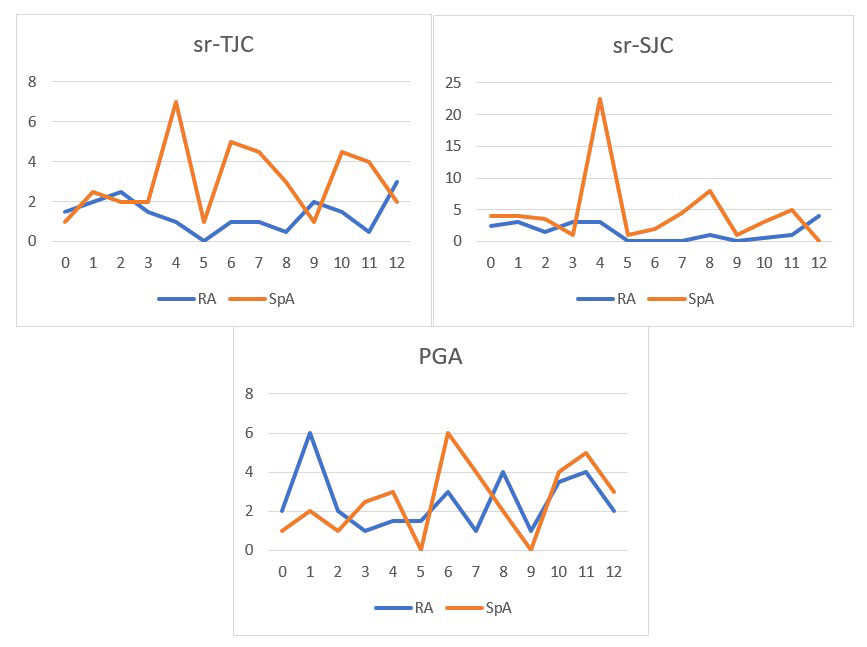
Results: Out of 46 recruited patients, 41 (18/22 (82%) RA, 23/24 (96%) SpA) downloaded and used the mobile solution and 37 (80%) submitted at least one ePROs entry. Mean (SD) age was 47 (13) and 43 (9) years in the RA and SpA groups, respectively. 14/18 (77%) patients with RA and 8/23 (35%) with SpA were female. ePROs measurements completion rates and overall results for RA and SpA patients that completed any data during follow-up are shown in Table 1. Follow-up results of ePROs that were assessed in both RA and SpA are shown in Figure 2. Patients with RA completed an average of 27 ePROs during follow-up, whereas patients with SpA completed a median of 16. Regarding alerts, 25 patients generated a total of 36 alerts, of which 32 were flares (17 RA, 15 SpA), 2 problems with medication and 2 infection/other. 26 (72.2%) of the alerts were managed remotely, 7 (19.4%) required a face-to-face intervention and in 3 (8.3%) did not respond before the consultation. Regarding usability and patient satisfaction, 34 patients provided telephonic qualitative feedback, and 14 responded to the online NPS. Overall acceptance was good: 33/34 patients acknowledged that they would recommend the solution, while 9/14 were considered promoters according to the NPS. The overall rating of these 14 patients for the app was 4.3 out of 5 stars.
Conclusion: This study shows that the use of a digital health solution is feasible in clinical practice. Based on these preliminary results, the next step will be to further implement this solution in a multicentric setting to analyze the added value for monitoring patients.
To cite this abstract in AMA style:
Benavent D, Fernández-Luque L, Sanz-Jardón M, Navarro-Compán V, Altur P, Calvo E, Lojo L, Balsa A, Plasencia-Rodriguez C. Remote Monitoring of Chronic Inflammatory Musculoskeletal Diseases– Results of the Digireuma Feasibility Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/remote-monitoring-of-chronic-inflammatory-musculoskeletal-diseases-results-of-the-digireuma-feasibility-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/remote-monitoring-of-chronic-inflammatory-musculoskeletal-diseases-results-of-the-digireuma-feasibility-study/