Session Information
Session Type: Abstract Session
Session Time: 8:00AM-9:00AM
Background/Purpose: The complement system is a major component of innate immunity and plays a vital role in experimental models of autoimmune arthritis pathogenesis. In patients with RA, local activations of the complement pathways in the synovium will interact with macrophages as well as other synovial mesenchymal cells that cause tissue inflammation. However, it remains to be determined how specific complement components in the RA synovium modulate cell type functions to both enhance injury but also strengthen phagocytosis and inflammation resolution.
Methods: AMP RA/SLE has generated a comprehensive synovial single-cell multimodal cell atlas ( >314,000 cells, 70 RA patients) and classified the spectrum of RA biopsies into six groups named CTAPs (Cell Type Abundance Phenotypes)1. In this study, we link complement activation pathways with cell-type heterogeneity, and test associations of complement components across different CTAPs using Generalized Linear Mixed Models. Furthermore, we characterize myeloid cell differentiation using single-cell trajectory analysis and align specific complement pathways to myeloid functional polarization states. Finally, we performed cell type interactome analysis to reveal complement-dependent receptor-ligand pairs among multiple cell types that likely contribute to tissue inflammation.
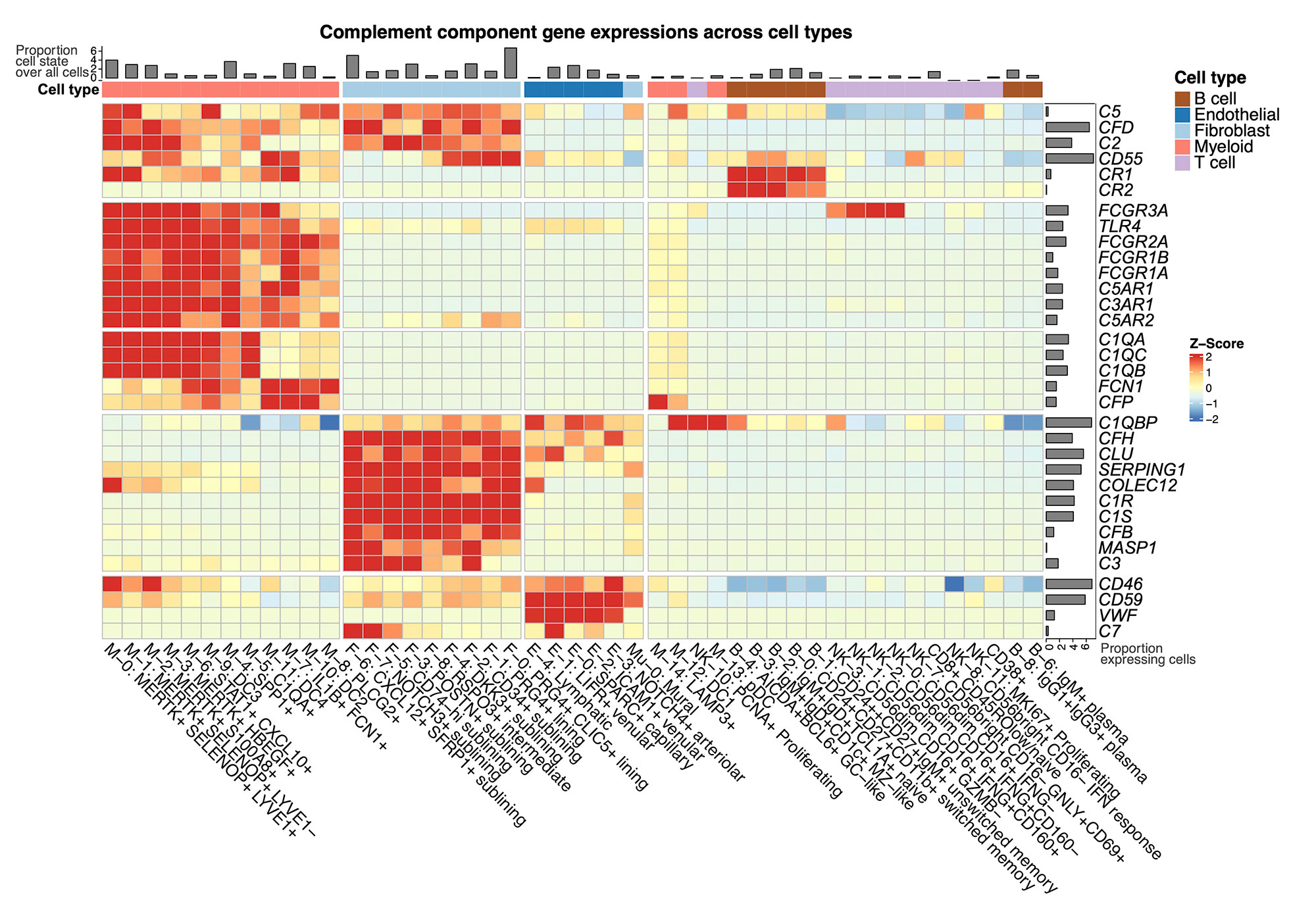
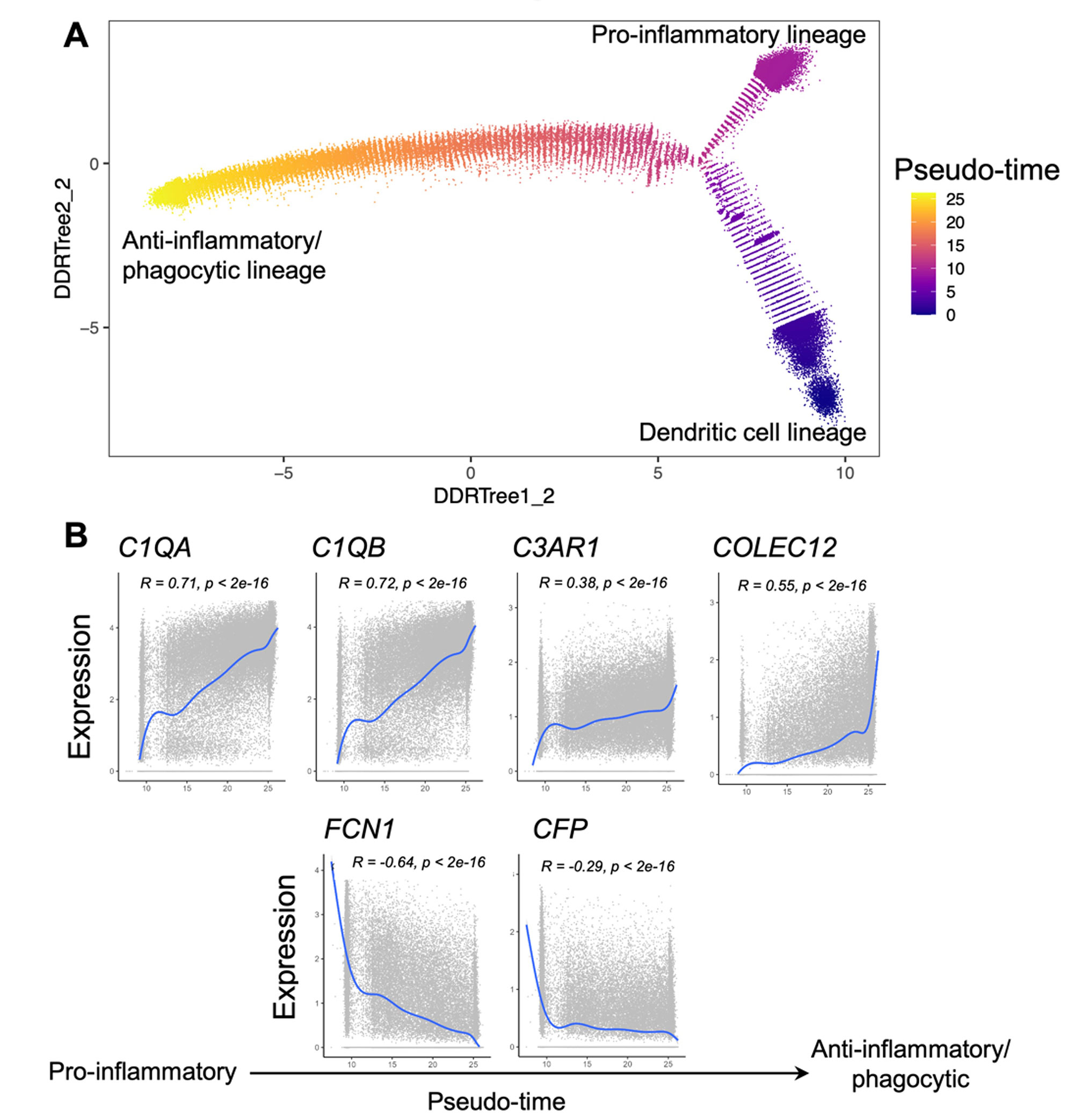
Results: We generated an Autoimmune Complement Cellular Graph (ACCG) comprehensively characterizing interactions between complement components and cell-type co-varying patterns in RA synovium. We found that complement components present distinct transcriptomic patterns across cell types in unexpected patterns (Fig. 1). Furthermore, each synovial CTAP contains a distinct selection of myeloid cell states. Specifically, expressions of complement C1QA-C, C3AR1, and COLEC12 are correlated with myeloid phagocytic trajectory, while complement FCN1 and CFP are correlated with myeloid pro-inflammatory trajectory, which suggests different complement pathways interact with myeloid states in RA synovium (Fig. 2). Intriguingly, the phagocytic MERTK+ tissue macrophages in CTAP-EFM (dominated by myeloid, fibroblast, and endothelial cells) display two complement pathways, namely the phagocytic C1QA-C factors and the inflammatory receptor C3AR1, while sublining fibroblasts highly expressed complement C3, which suggests the potential interactions between MERTK+ macrophages and specific fibroblasts that activate complement pathways in the synovium.
Conclusion: Through systematically aligning complement pathways with synovial cellular heterogeneity, we generated a map describing how specific complement components can modulate functional myeloid states to strengthen resolution in RA patients through phagocytic functions. This approach of analyzing high-resolution single-cell multimodal data in human tissues with a focus on the complement system brings insights into new complement pathway-based cellular targets for patients with RA, and provides a roadmap for similar studies of other complex pathways in tissues.
To cite this abstract in AMA style:
Vargas J, Banda N, Mantel I, Jonsson A, Wei K, Rao D, Goodman S, Deane K, Seifert J, Anolik J, Brenner M, Raychaudhuri S, RA/SLE A, Holers M, Donlin L, Zhang F. Deciphering Complement System-dependent Cellular Pathways in Human Rheumatoid Arthritis Synovial Tissues Using Single-cell Computational Omics [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/deciphering-complement-system-dependent-cellular-pathways-in-human-rheumatoid-arthritis-synovial-tissues-using-single-cell-computational-omics/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deciphering-complement-system-dependent-cellular-pathways-in-human-rheumatoid-arthritis-synovial-tissues-using-single-cell-computational-omics/