Session Information
Date: Saturday, November 12, 2022
Title: Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Sites of peripheral joint and enthesitis involvement in AS vary. Tofacitinib is an oral Janus kinase inhibitor for the treatment of AS. Differential site-specific treatment responses in the joints have been observed with tofacitinib and adalimumab in patients (pts) with RA1 and PsA.2 Real-world data of pts with axial SpA also showed site-specific treatment responses in the entheses with a TNF inhibitor.3 We assessed the site-specific responses of joint and entheses to tofacitinib in pts with active AS.
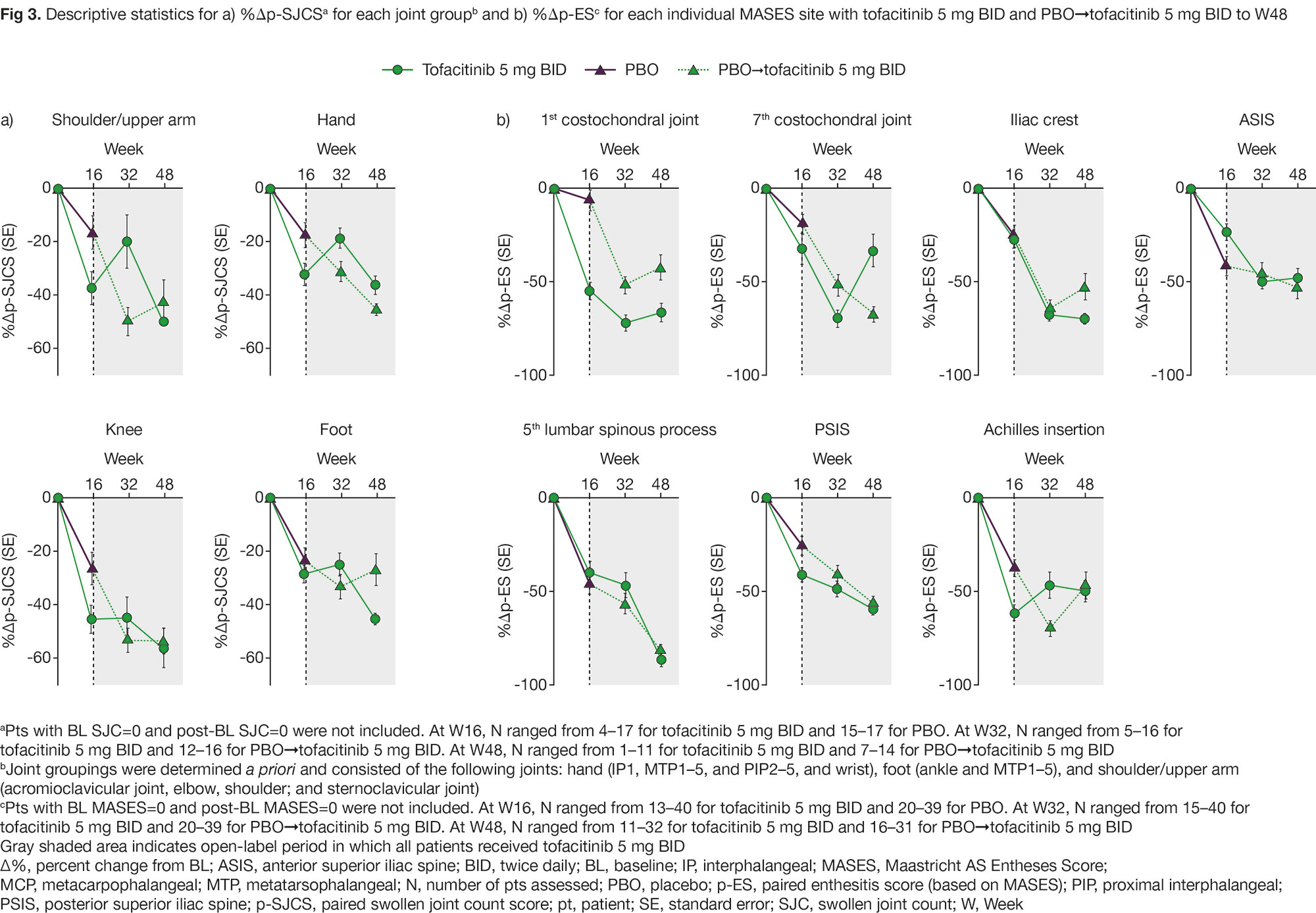
Methods: This post hoc analysis used data up to Week (W)48 from a Phase 3 study (NCT03502616; N=269) in pts with active AS, fulfilling the modified New York criteria, and had an inadequate response/intolerance to ≥ 2 NSAIDs. Pts received tofacitinib 5 mg twice daily (BID) or placebo (PBO) for 16 weeks; from W16, all pts received open-label tofacitinib 5 mg BID to W48. A paired swollen joint count score (p-SJCS) and a combination of bilateral SJC ranging from 0 (neither side swollen) to 2 (both sides swollen) was calculated. Baseline (BL) joint involvement frequencies, mean p-SJCS (for pts with BL SJC ≥ 1) and percent change from BL (%Δ) in p-SJCS for each paired joint/paired joint group (hand, shoulder/upper arm, and foot), were calculated. A paired enthesitis score (p-ES) was derived from the Maastricht AS Enthesitis Score (MASES) sites. BL enthesitis involvement frequencies, mean p-ES (for pts with BL MASES ≥ 1) and %Δp-ES were calculated.
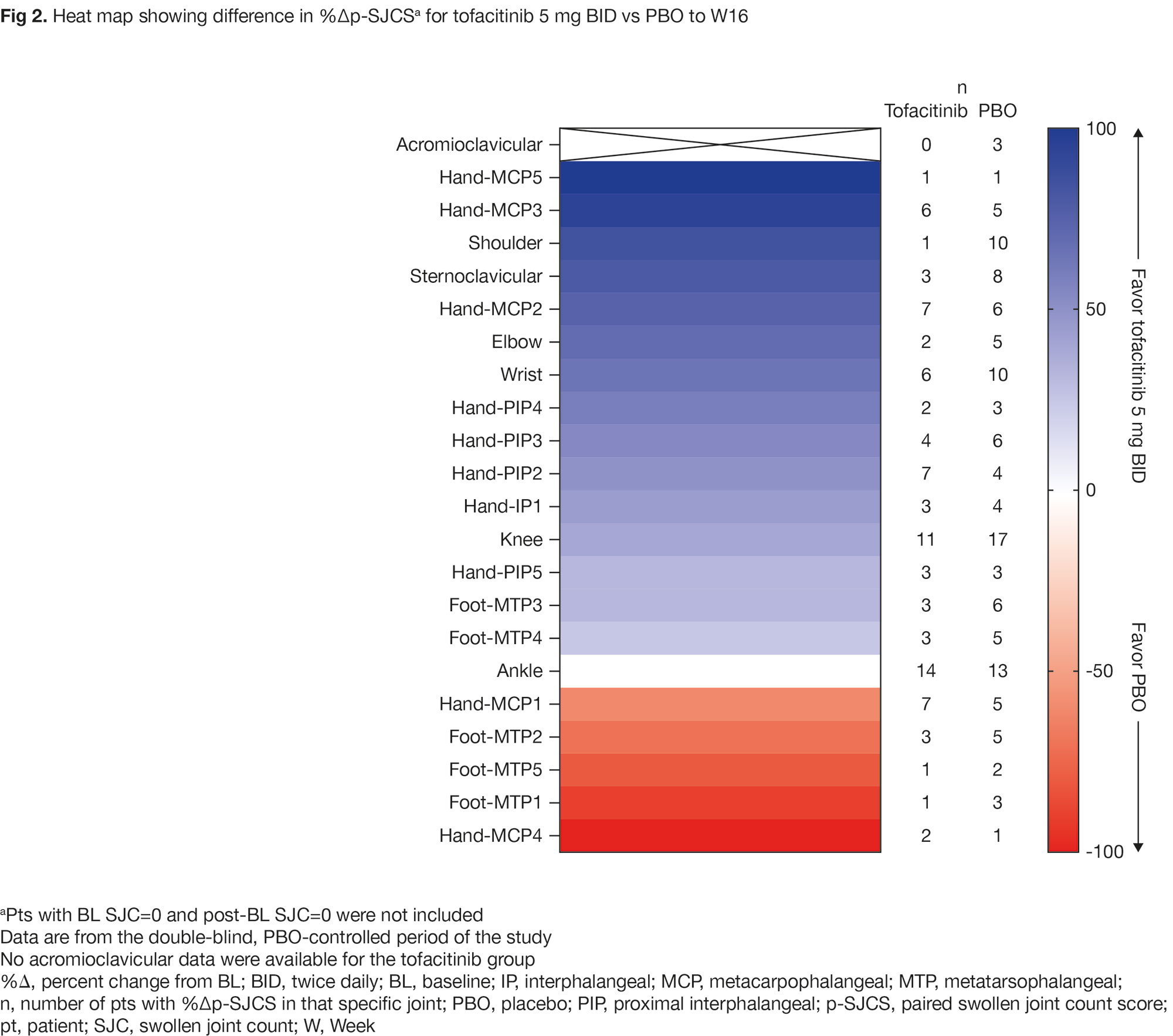
Results: Of note, at BL, frequency of joint involvement in the shoulder/feet was lower with tofacitinib vs PBO; mean p-SJCS in the feet were lower with tofacitinib vs PBO (Fig 1a). Frequency of enthesitis involvement at BL at the 7th costochondral (CC) joint/Achilles tendon was lower with tofacitinib vs PBO (Fig 1b). At W16, greater reduction in %Δp-SJCS was observed with tofacitinib vs PBO in most joints (Fig 2) and across joint groups, except the foot (Fig 3a). At most enthesitis sites, tofacitinib and PBO elicited broadly similar %Δp-ES up to W16; however, greater responses at W16 were observed at the 1st CC joint/Achilles tendon with tofacitinib vs PBO (Fig 3b). To W48, p-SJCS/p-ES were generally reduced in both treatment groups (Fig 3). Limitations of this exploratory analysis include low number of pts with BL SJC/MASES and the dichotomous nature of SJC/MASES.
Conclusion: In pts with AS, we generally observed BL differences in peripheral joint and enthesis involvement and site-specific responses between tofacitinib and PBO. A BL imbalance of arthritis frequency/severity in the feet may have led to a floor effect with tofacitinib, though a relatively poorer tofacitinib response in the feet vs other joint groups has previously been reported in RA1/PsA2 and swelling in the foot, including the MTP joint, may be difficult to assess. Decreases from BL in p-ES were observed at most sites with both tofacitinib and PBO, with better responses at the 1st CC joint/Achilles tendon at W16 with tofacitinib vs PBO.
1. Frank-Bertoncelj et al. Arthritis Rheumatol 2019; 71 (Suppl 10): Abs 1338
2. Ciurea et al. Arthritis Rheumatol 2021; 73 (Suppl 10): Abs 1349
3. Nissen et al. Arthritis Res Ther 2021; 23: 165
Study sponsored by Pfizer. Medical writing support was provided by J Juana, CMC Connect, and funded by Pfizer.
To cite this abstract in AMA style:
Micheroli R, Killeen T, Jo H, Kwok K, Elzorkany B, Ospelt C, Ciurea A, Nissen M. Site-Specific Responses of Joint and Entheses to Tofacitinib in Patients with Ankylosing Spondylitis: A Post Hoc Analysis of a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/site-specific-responses-of-joint-and-entheses-to-tofacitinib-in-patients-with-ankylosing-spondylitis-a-post-hoc-analysis-of-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/site-specific-responses-of-joint-and-entheses-to-tofacitinib-in-patients-with-ankylosing-spondylitis-a-post-hoc-analysis-of-a-phase-3-trial/