Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Brepocitinib is a novel, orally available, TYK2/JAK1 inhibitor in Phase 3 development for the treatment of dermatomyositis (DM), a chronic immune-mediated disease of the skin and muscles. Pathobiologically, DM is primarily driven by Type I interferon (IFN-I) dysregulation. Importantly, TYK2 and JAK1 are essential to the signaling of IFN-I and other cytokines (e.g., IL-12, IL-23) implicated in the pathogenesis of DM. Several FDA-approved JAK inhibitors (JAKi) have been evaluated off-label in case reports and pilot studies in DM and have shown clinically meaningful efficacy and acceptable safety profiles in >140 patients (Paik et al, Clin Exp Rheumatol, in press). These observations, along with the clinical pharmacokinetic (PK) and pharmacologic analyses conducted and described here, support the rationale and dose selection for a well-controlled Phase 3 study evaluating the safety and efficacy of brepocitinib in DM.
Methods: Brepocitinib’s inhibition of human JAKs was assessed via recombinant enzyme inhibition assays and cellular potency. Selectivity was quantified via inhibition of cytokine-induced STAT phosphorylation in human whole blood. Human PK data was used to estimate the expected in vivo suppression of cytokine signaling pathways implicated in DM pathogenesis based on daily average blood brepocitinib concentrations (Cavg). A cross-study dose-response analysis (Emax model fit to normalized responder rate) also was conducted with 5 completed, placebo-controlled, Phase 2 studies (psoriatic arthritis, N=218, NCT03963401; plaque psoriasis, N=212, NCT02969018; ulcerative colitis, N=167, NCT02958865; alopecia areata, N=94, NCT02974868; and hidradenitis suppurativa, N=100 NCT04092452).
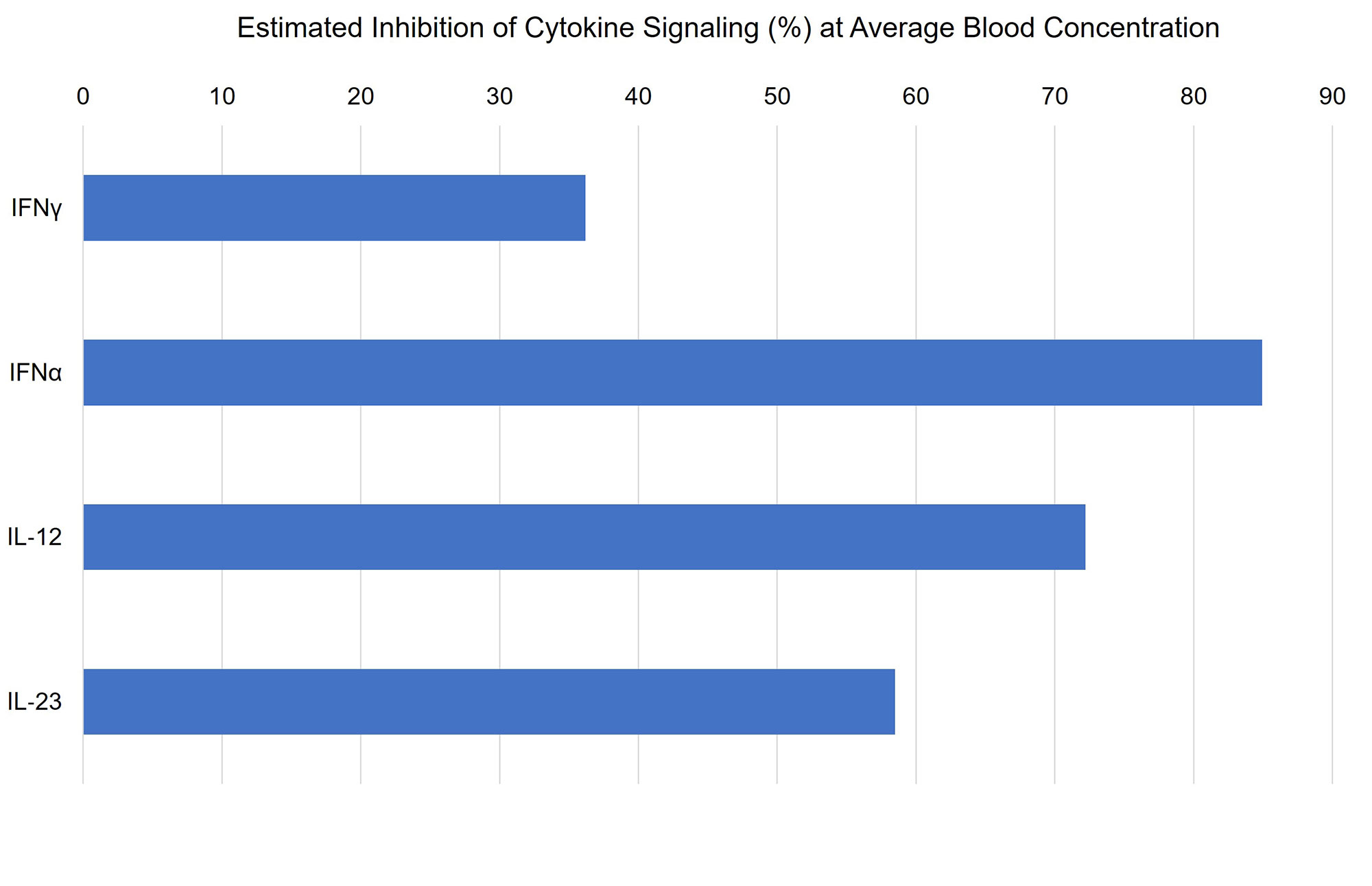
Results: In vitro, brepocitinib potently and selectively inhibits JAK1 and TYK2, with IC50 values (23 and 17 nM, respectively) at least 3-fold lower than those for JAK2 and JAK3. In human whole blood, brepocitinib inhibited cytokine signaling pathways implicated in DM that engage TYK2 and/or JAK1, including IFNα, IFNg, IL-12, and IL-23. Estimated inhibition of these cytokine signaling pathways at the Cavg of brepocitinib 30 mg once daily (QD) is shown in Figure 1. Across Phase 2 studies, brepocitinib demonstrated dose-dependent efficacy. The relationship between normalized responder rate and average daily brepocitinib dose is shown in Figure 2. The dose resulting in 50% maximal response was determined to be 15 mg QD with generally minimal increased efficacy observed at doses greater than 30 mg QD.
Conclusion: There is strong clinical evidence supporting JAKi for the treatment of DM. Several JAKi have demonstrated efficacy profiles in other autoimmune diseases similar to those observed for brepocitinib 30 mg QD shown here. In addition, brepocitinib is unique amongst the JAKi class for its potent dual inhibition of TYK2 and JAK1 and is therefore well suited to address the cytokine signaling dysregulations implicated in the pathogenesis of DM (particularly IFN-I), with relevant inhibition expected to occur in a clinically relevant dose range. Collectively, these analyses support the evaluation of brepocitinib 15 and 30 mg QD for the treatment of DM in a robust, well-controlled, Phase 3 study.
The response rate for each endpoint in each study was normalized to the maximum response rate for that endpoint, which was given the nominal response rate of 100%.
Abbreviations: SALT(30, 50, 75, 90, 100) = ≥ 30%, 50%, 75%, 90%, 100% regrowth compared with baseline using the Severity of Alopecia Tool; HiSCR = Hidradenitis Suppurativa Clinical Response; PASI75 = 75% reduction in the Psoriasis Area and Severity Index score; ACR(20, 50, 70) = ≥ 20%, 50%, 70% improvement in American College of Rheumatology core set measures.
To cite this abstract in AMA style:
Aggarwal R, Johnson B, Feldman J, Gromatzky A, Mudd Jr. P. Brepocitinib for the Treatment of Dermatomyositis: Pharmacologic and Clinical Rationale [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/brepocitinib-for-the-treatment-of-dermatomyositis-pharmacologic-and-clinical-rationale/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/brepocitinib-for-the-treatment-of-dermatomyositis-pharmacologic-and-clinical-rationale/