Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: The synovium envelopes the diarthodial joint and is composed of fibroblast-like synoviocytes (FLS) and macrophage-like synoviocytes (MLS). In healthy joints, the synovial cavity maintains sterility and the interplay between FLS and MLS contributes to synovial homeostasis. In the context of osteoarthritis (OA), however, the synovium undergoes marked structural and compositional changes, characterized by leukocyte infiltration and fibrotic remodeling of the synovial matrix. While soluble factors such as cytokines are known to regulate the behavior of FLS and MLS during OA pathogenesis, less is known regarding how biophysical cues regulate the individual response and paracrine interactions between these two cell types. Recent studies show that macrophages (Mϕ) are mechanosensitive and tune their inflammatory phenotypes in response to altered microenvironmental mechanics1. Here, we test the hypothesis that fibrotic remodeling and stiffening of the synovium impacts the inflammatory secretome of synovial Mϕs, which in turn modulates FLS behavior.
Methods: IHC: Paraffin sections of human synovial tissue [n=4 healthy organ donors (OD); n=5 total knee arthroplasty patients (TKA)] were stained for H&E or DAPI, CCR7, CD68, PRG4, and p65. Mϕ Secretome Analyses: 5 and 55 kPa polyacrylamide (PA) gels were fabricated and functionalized with fibronectin. THP-1 Mϕs were seeded on gels and cultured with or without 50ng/mL TNF-ɑ for 24 hours. RNA was isolated for qPCR. Conditioned media from THP-1 Mϕs was collected and applied for 24 hours to human mesenchymal stem cells (hMSCs) cultured on tissue culture plastic. hMSCs were used as a surrogate mesenchymal cell type to represent FLS. RNA was isolated from hMSCs for qPCR.
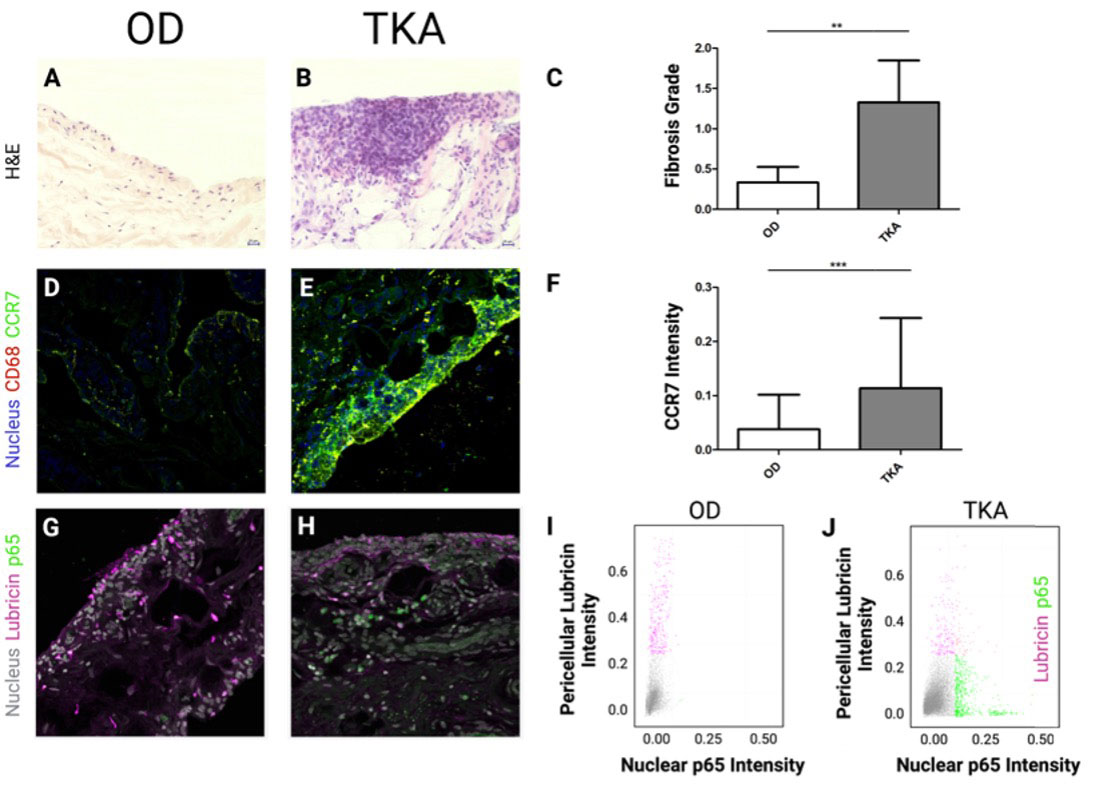
Results: TKA synovia were highly fibrotic, enlarged with hyperplasia (Fig 1A, B, C) and exhibited higher fluorescence intensity for CCR7 compared to OD tissue (Fig 1D, E, F). Single-cell analysis revealed that in OD synovia, FLS stained positive for PRG4 (lubricin) but not for NF-????B/p65 (Fig 1G,H,I). Conversely in TKA synovia, p65 signaling was apparent along with a marked decrease in PRG4 expression (Fig 1J).
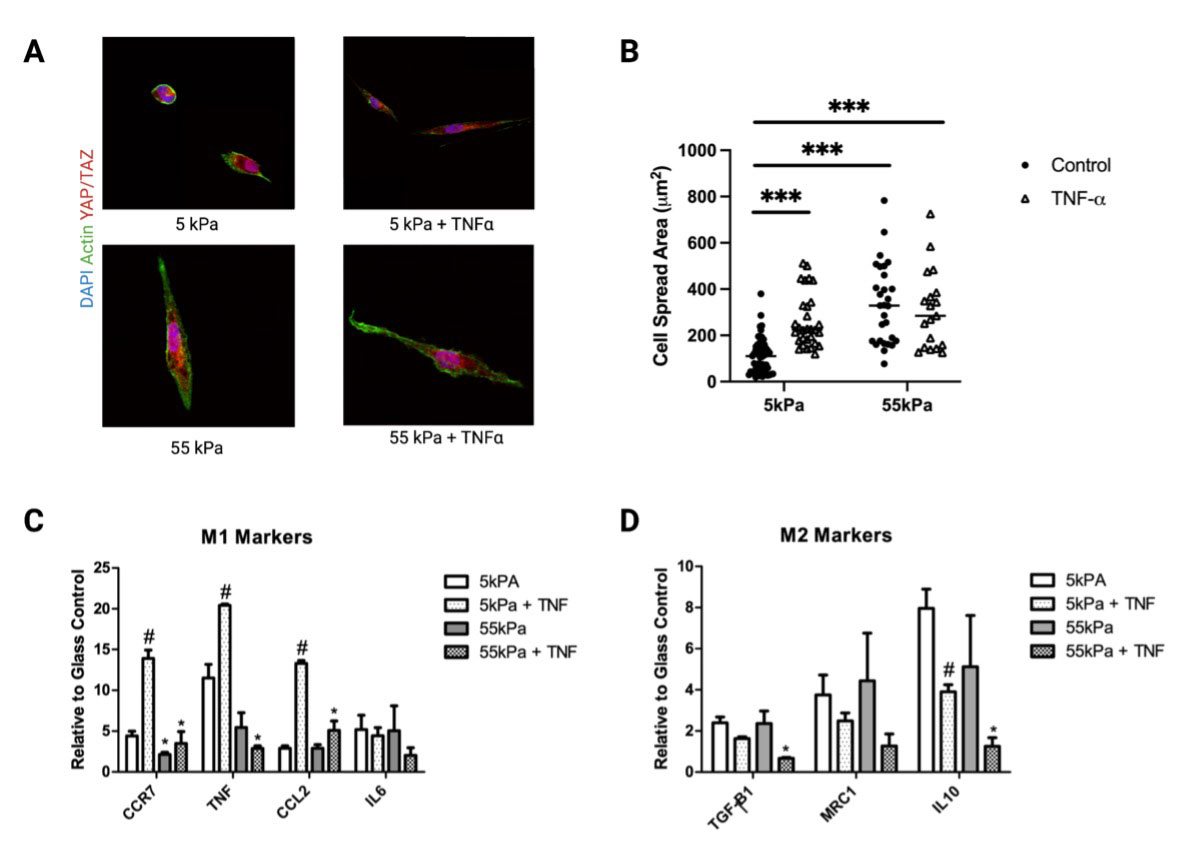
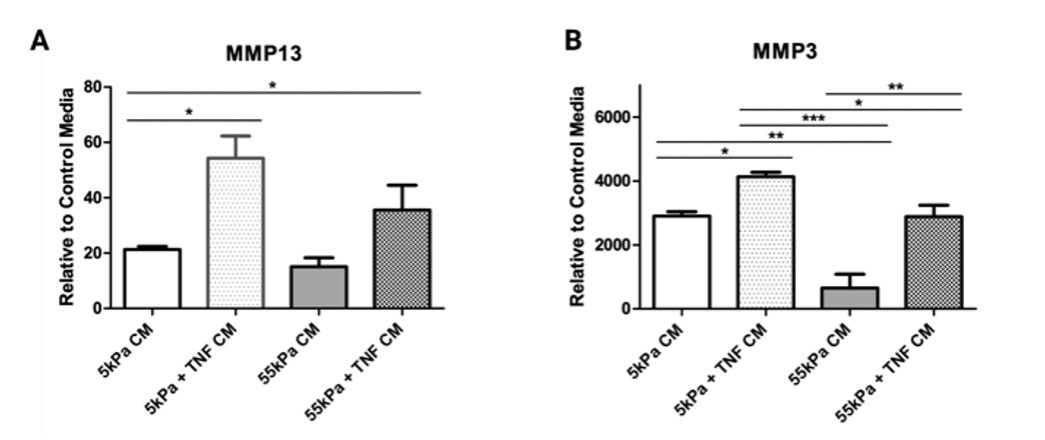
Mϕs cultured without TNF-ɑ exhibited moduli-dependent changes in cell morphology (Fig 2A) and TNF-ɑ increased cell spreading at lower stiffness (Fig 2B). Expression of M1 markers increased substantially for THP-1 Mϕs on soft substrates in the presence of TNF-ɑ (Fig 2C). For M2 markers, IL-10 expression was significantly reduced on 55kPa compared to 5kPa in the presence of TNF-ɑ (Fig 2D). Conditioned media from THP-1 cultured on 5kPa with TNF-ɑ induced the greatest inflammatory activation of hMSCs (Fig3A, B).
Conclusion: Microenvironmental mechanics and biochemical signals integrate to modulate Mϕ polarization, which in turn affects Mϕ communication with surrounding cells. We found that adhesion to soft hydrogels increases the inflammatory response of Mϕs compared to stiff substrates. Moreover, the secretome of Mϕs cultured on soft substrates increased the catabolic and inflammatory activation of hMSCs. This sheds light on a possible feed-forward cascade of disease, where the changing biophysical environment promotes aberrant phenotypic changes.
To cite this abstract in AMA style:
Kim S, Scanzello C, Mauck R, Bonnevie E. Microenvironmental Stiffness Modulates Macrophage Responsiveness and Communication with Mesenchymal Stem Cells [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/microenvironmental-stiffness-modulates-macrophage-responsiveness-and-communication-with-mesenchymal-stem-cells/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/microenvironmental-stiffness-modulates-macrophage-responsiveness-and-communication-with-mesenchymal-stem-cells/