Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Upadacitinib (UPA) is a Janus kinase inhibitor that has been shown to be effective and well tolerated in patients with rheumatoid arthritis (RA) in the Phase 3 SELECT clinical trials.1–6 However, data on the use of UPA in real-world clinical practice are limited. The UPwArds study aims to investigate the effectiveness and safety of UPA as monotherapy or in combination with methotrexate (MTX) in patients with RA in German clinical practice.
Methods: UPwArds is an ongoing, prospective, multicenter, non-interventional, post-marketing study in adult patients with moderate-to-severe RA who are treated with UPA 15 mg once daily according to the product label, with the decision to initiate UPA made prior to study participation. Per protocol, the study enrolled around 50% of patients receiving UPA monotherapy and 50% of patients receiving UPA + MTX. The primary endpoint was the proportion of patients achieving remission defined as Clinical Disease Activity Index (CDAI) ≤2.8 at 6 months. Key secondary endpoints included the proportion of patients achieving CDAI ≤2.8 and ≤10, Simple Disease Activity Index (SDAI) ≤3.3 and ≤11, and 28-joint Disease Activity Score using CRP (DAS28[CRP]) < 2.6 and ≤3.2 at each visit. Changes from baseline in disease activity and patient-reported outcomes were also assessed. This descriptive interim analysis reports data for the patients who had completed Visit 3 (Month 6) by the cut-off date of March 22, 2021. Data are presented as observed and summarized descriptively, with no statistical analyses conducted.
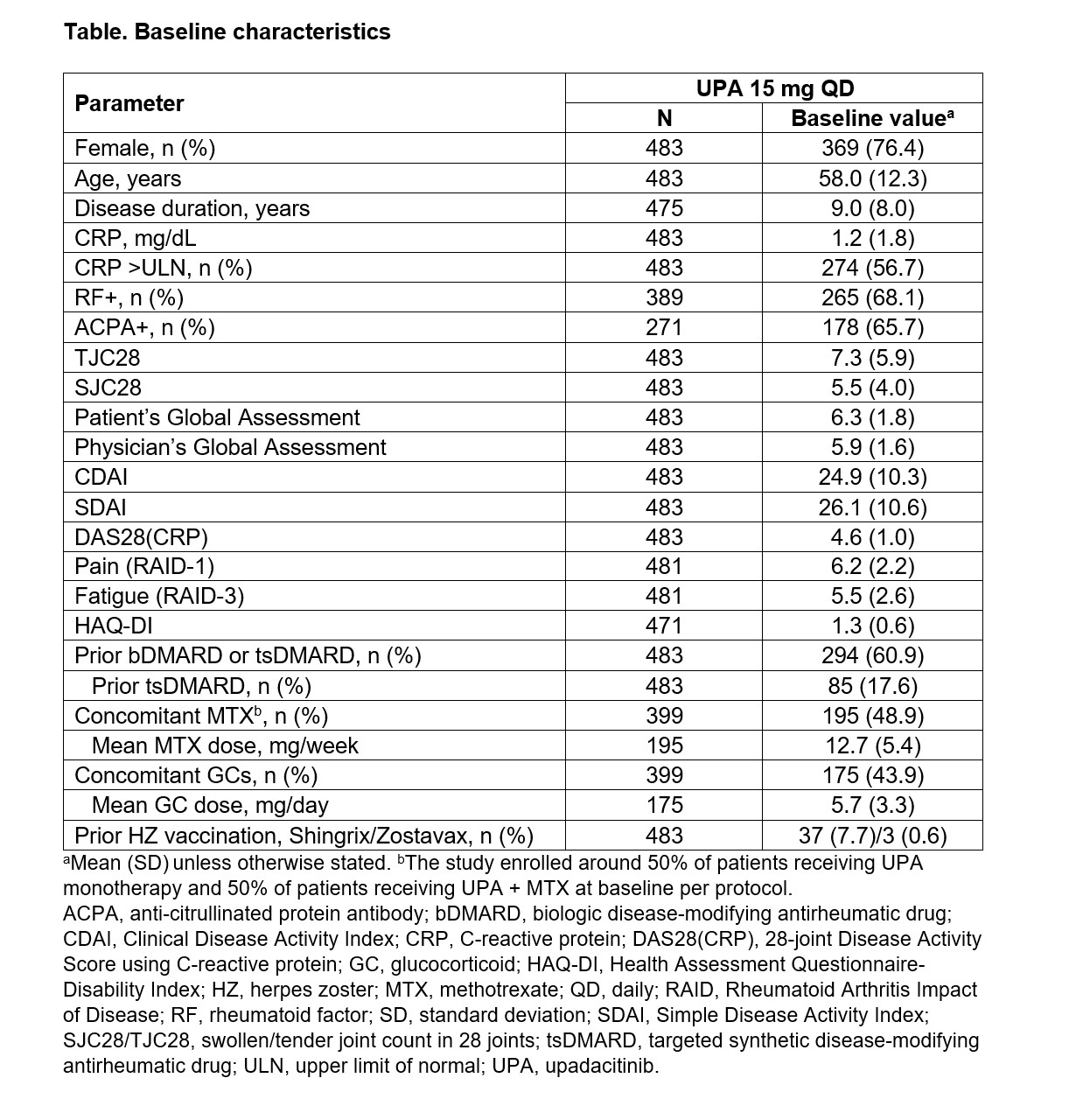
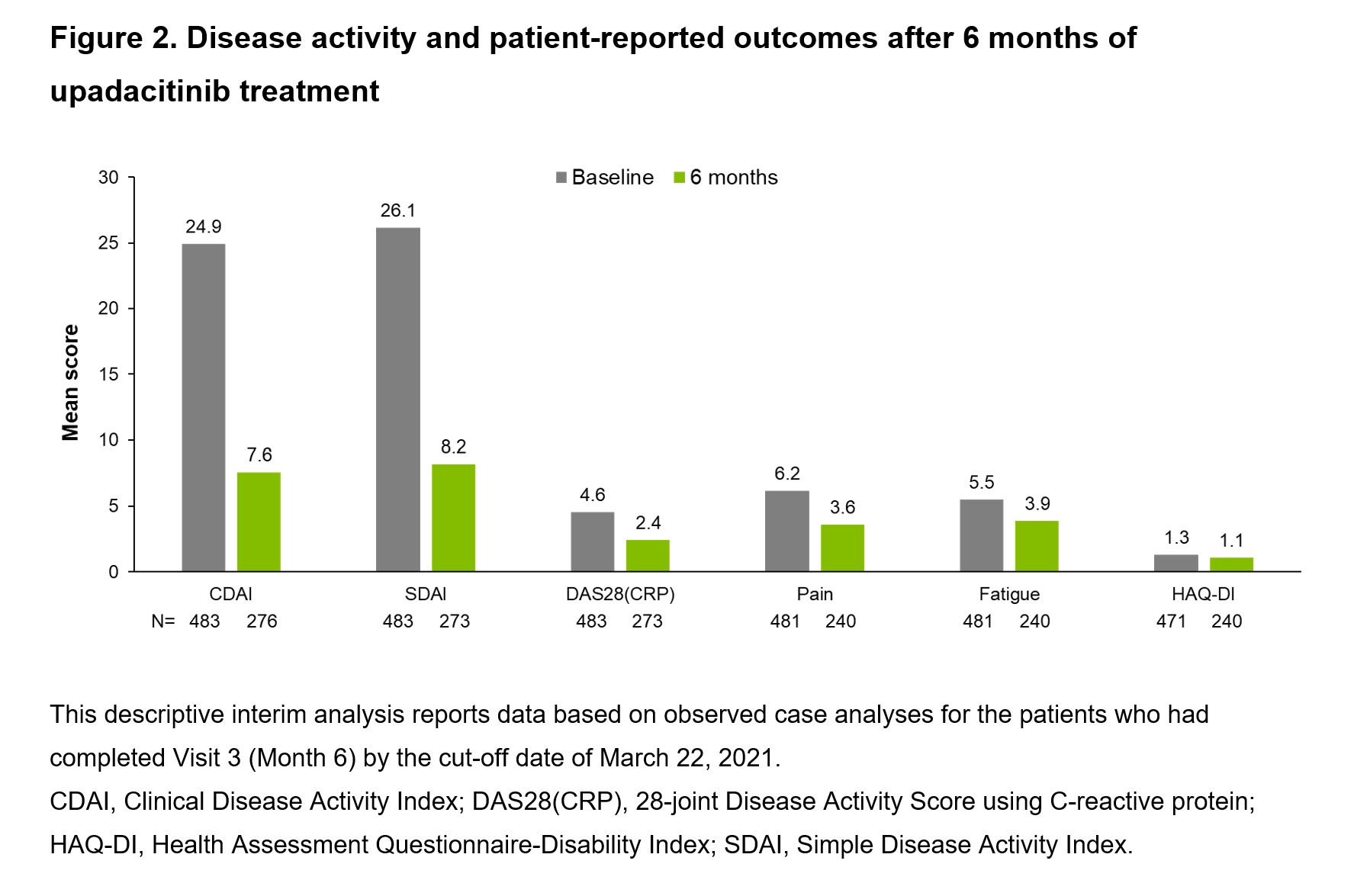
Results: Of the 483 patients at baseline (76.4% female; mean disease duration: 9 years), 60.9% had previously been treated with biologic (b) or targeted synthetic (ts) DMARDs (Table 1). Of the patients with 6-month data, 25.4% (70/276) and 74.6% (206/276) achieved CDAI remission (primary endpoint) and LDA, respectively, after 6 months of UPA 15 mg treatment (Figure 1). DAS28(CRP) < 2.6 and ≤3.2 at Month 6 were achieved by 65.2% (178/273) and 79.1% (216/273) of patients, respectively. The percentage of patients achieving any definition of LDA (DAS28[CRP] ≤3.2, CDAI ≤10, or SDAI ≤11) or remission (DAS28[CRP] < 2.6, CDAI ≤2.8, or Boolean remission) at 6 months was similar among those treated with UPA monotherapy (72.9%) or UPA + MTX (74.1%). Disease activity and patient-reported outcomes (including pain, fatigue, and physical function) improved within the first 6 months following initiation of UPA 15 mg (Figure 2). The safety profile of UPA 15 mg was consistent with that seen in the Phase 3 trials with no new safety signals.1–6
Conclusion: Consistent with previous clinical data, the 6-month interim results from this study suggest that UPA as monotherapy or in combination with MTX is associated with a favorable benefit–risk profile in real-world patients with RA.
- Smolen JS, et al. Lancet 2019;393:2303–11.
- Burmester GR, et al. Lancet 2018;391:2503–12.
- Genovese MC, et al. Lancet 2018;391:2513–24.
- van Vollenhoven R, et al. Arthritis Rheumatol 2020;72:1607–20.
- Fleischmann R, et al. Arthritis Rheumatol 2019;71:1788–800.
- Rubbert-Roth A, et al. N Engl J Med 2020;383:1511–21.
To cite this abstract in AMA style:
Witte T, Kiltz U, Haas F, Riechers E, Prothmann U, Adolf D, Holland C, Hecht R, Rössler A, Famulla K, Götz K, Krüger K. Effectiveness of Upadacitinib in Patients with Rheumatoid Arthritis in German Real-World Practice: Interim Results from a Post-Marketing Observational Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-upadacitinib-in-patients-with-rheumatoid-arthritis-in-german-real-world-practice-interim-results-from-a-post-marketing-observational-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-upadacitinib-in-patients-with-rheumatoid-arthritis-in-german-real-world-practice-interim-results-from-a-post-marketing-observational-study/