Session Information
Session Type: Abstract Session
Session Time: 10:45AM-11:00AM
Background/Purpose: We have previously shown that decreased expression of the deacetylase sirtuin-1 (SIRT1) contributes to the proliferative, activated and proangiogenic profile of endothelial cells (EC) in rheumatoid arthritis (RA). The matricellular protein CCN1, characterized by proangiogenic and immunomodulatory properties, may be directly implicated in these processes, since its expression is negatively regulated by SIRT1.Our objective was to study the implication of CCN1 in RA pathogenesis.
Methods: CCN1 expression was assessed in ECs (25 RA and 10 controls) by quantitative RT-PCR, western blot and ELISA, in the synovial tissue (5 RA and 5 controls) by immunohistochemistry and immunofluorescence, and in the serum (205 RA and 20 controls) by ELISA. Invalidation of CCN1 in RA ECs was achieved through the use of shRNA and neutralizing monoclonal antibodies. The functional consequences of CCN1 invalidation in RA ECs were studied i) in vitro by the analysis of proliferation (cell impedance), tube formation in Matrigel and migration in Boyden chambers; and ii) in vivoin the murine model of tumor neoangiogenesis. Conditional invalidation of CCN1 in ECs was analyzed in the mouse model of methyl-BSA-induced arthritis.
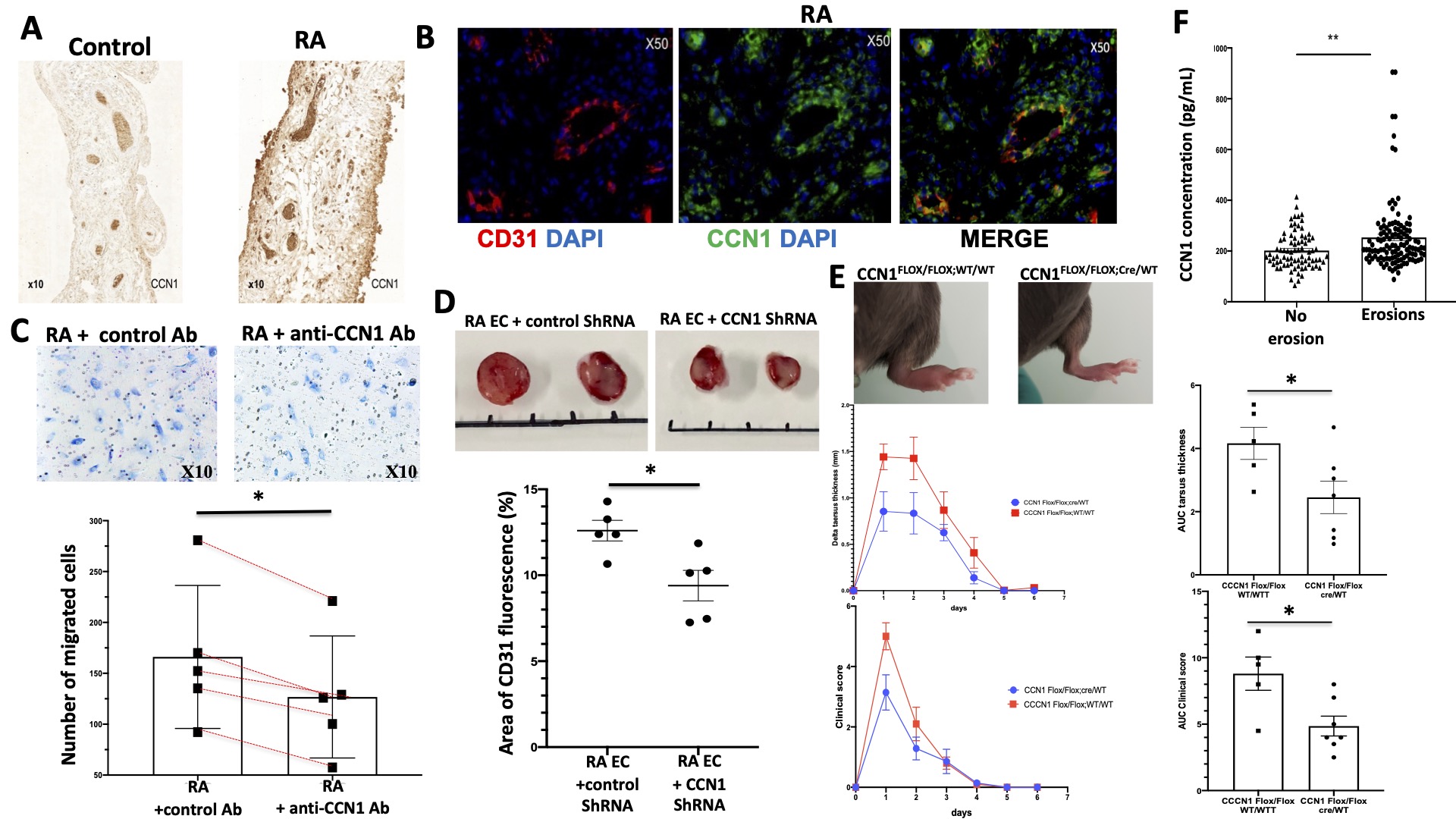
Results: CCN1 mRNA and protein expression were increased by 1.72- (p = 0.012) and 7.2-fold (p=0.008) in RA ECs compared to controls, respectively. CCN1 concentrations were significantly increased in RA EC culture supernatants (930±153 vs. 359±199 pg/mL, p=0.007). CCN1 was overexpressed in the synovial tissue of RA patients (Figure 1A) and confocal microscopy analyses revealed a prominent CCN1 expression in the vascular endothelium (CD31 +) and T cells (CD3 +) (Figure 1B). In vitro, recombinant TNF-α and IL-17 induced the mRNA and protein expression of CCN1 in RA ECs. CCN1 invalidation was associated with reduced proliferative capacities, delayed capillary tube formation, and decreased migration (Figure 1C) of RA ECs. In vivo, subcutaneous transplantation of CT26 tumor cells combined with RA ECs transfected with CCN1 shRNA to CB17 SCID mice was associated with a 51% reduction in tumor volume (p=0.008) and a 27% reduction in tumoral vascular density (p=0.032) compared with mice transplanted with MOCK transfected RA-ECs (Figure 1D). Conditional deletion of CCN1 in ECs through a Cre-LoxP recombination system alleviated signs of methyl-BSA-induced arthritis (Figure 1E). Serum CCN1 concentrations were significantly higher in the presence of bone erosions (253±139 vs. 202±7 pg/mL, p=0.002) (Figure 1F) and correlated with radiographic Larsen score (r=0.3, p=0.001) and HAQ (r=0.25, p=0.012).
Conclusion: CCN1 is overexpressed in ECs and the synovial tissue of patients with RA. CCN1 also regulate the functional properties of RA ECs and their angiogenic potential in vivo. Moreover, endothelial inactivation of CCN1 alleviate experimental arthritis. CCN1 could represent a new therapeutic target, which is being evaluated in experimental models of erosive arthritis. CCN1 may also be a reliable biomarker of structural damages given the association between its serum concentrations and the extent of radiographic lesions. The performance of CCN1 serum levels to predict structural progression is under investigation.
To cite this abstract in AMA style:
Avouac J, Steelandt A, Amiar O, Leblond A, Orvain C, Cauvet A, Gonzalez V, Allanore Y. CCN1: An Angiogenic Actor Implicated in the Structural Damages of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/ccn1-an-angiogenic-actor-implicated-in-the-structural-damages-of-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ccn1-an-angiogenic-actor-implicated-in-the-structural-damages-of-rheumatoid-arthritis/