Session Information
Date: Saturday, November 6, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster I: Diagnosis (0323–0356)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: The comparative clinical utility of various diagnostic tests to aid the differential diagnosis of SLE is unclear. We compare the outcomes of testing with a multianalyte assay panel (MAP) with cell-bound complement activation products vs. standard of care lab testing in patients suspected of having SLE.
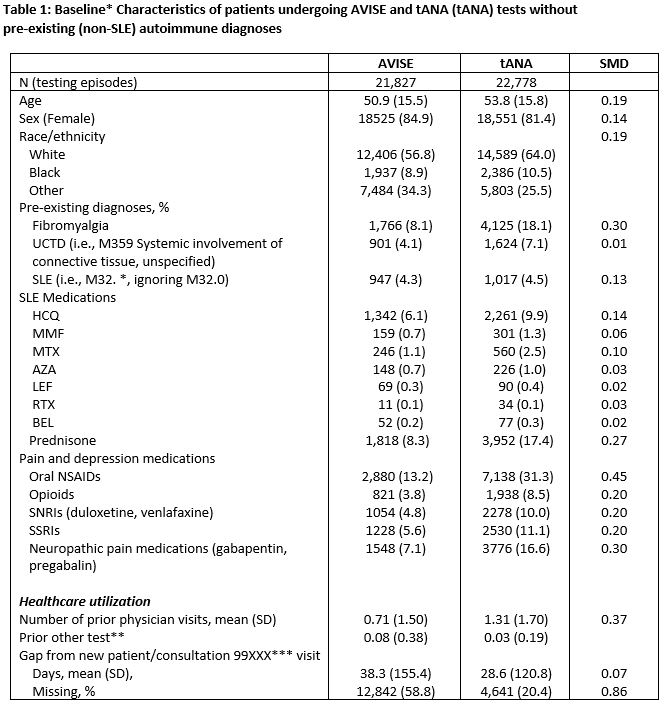
Methods: We used data from the electronic health record (EHR)-based Columbus registry from 1/2016 to 12/2020 for analysis and linked Healthcore medical, pharmacy, and claims data. We established two cohorts for comparison, the MAP testing strategy cohort and the traditional ANA testing strategy cohort (tANA), with or without anti-dsDNA or anti-Smith. All lab results from the MAP cohort were obtained directly from the lab vendor. Results from the tANA cohort were available from within the EHR. Baseline data was collected for both cohorts at 12 months (or more) before first testing. Using this data, we characterized the demographics, comorbidities, other autoimmune diagnoses, and medications (including SLE treatments and glucocorticoids). Except for SLE (which might have been only a working diagnosis), patients with pre-existing rheumatology conditions already identified at time of testing were excluded. Descriptive statistics compared the characteristics of MAP and tANA testing episodes. Logistic regression was used to estimate odds ratios (OR) comparing the likelihood of SLE medication initiation and SLE diagnosis between the MAP and tANA cohorts. Multivariable adjustment was performed and included all imbalanced covariates described in table 1 with standardized mean difference (SMD) >0.10. A subgroup analysis was conducted in patients new to the practice in the preceding 12 months
Results: The MAP and tANA cohorts consisted of 21,827 and 22,778 testing episodes, respectively. Patients in both cohorts were of similar age, mean 51 and 54 years, respectively for MAP and tANA, predominantly female (85 and 81%), and majority white (57 and 64%). Approximately 4-5% of MAP and tANA patients already had a SLE diagnosis ICD-10 code prior to testing. A total of 2,437 (11.17%) patients tested positive by MAP compared to 5,364 (23.55%) of patients who tested positive by tANA.
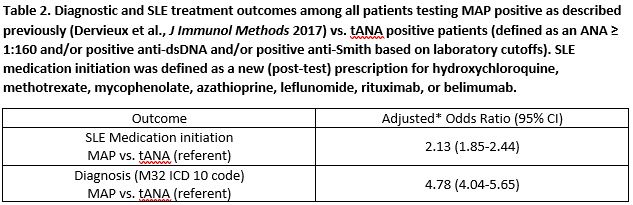
Among patients with no baseline prescription for a SLE medication, MAP positive patients were more likely to initiate SLE medications compared to tANA positive patients (43% vs. 32%, adjusted OR = 2.13, 95% CI 1.85-2.44). Effects were stronger in the subgroup of patients new to the practice, 55% vs. 33% (adjusted OR = 2.77, 95% CI 2.31-3.32). MAP positive patients were approximately fivefold more likely to be diagnosed with SLE as compared to the tANA positive patients, 31 vs. 8% (adjusted OR = 4.78, 95% CI 4.04-5.65); similar results were observed in the subgroup of new patients (adjusted OR = 6.34, 95% CI 5.12-7.86). Among MAP algorithm negative patients, 96% were not diagnosed with SLE compared to 99% of tANA negative patients.
Conclusion: Positive MAP results associated with a greater likelihood to initiate SLE medications and to receive a SLE diagnosis compared to tANA while maintaining comparable negative predictive value. The findings highlight the superior actionability of MAP results vs. tANA.
†For AVISE group is the tANA, for tANA is the AVISE
‡New patient ambulatory codes: 99201-5; new consultation codes: 99241-5
tANA – traditional antinuclear antibody; SLE – systemic lupus erythematosus; UCTD – undifferentiated connective tissue disease; HCQ – hydroxychloroquine; MMF – mycophenolate; MTX – methotrexate; AZA – Azathioprine; LEF – leflunomide; RTX – rituximab; BEL – Belimumab; NSAID – non-steroidal anti-inflammatory; SNRI – serotonin-norephinephrine reuptake inhibitors; SSRI – selective serotonin reuptake inhibitor; SMD = standardized mean difference (SMDs > 0.10 are considered potentially clinically important).
To cite this abstract in AMA style:
O'Malley T, Xie F, Su y, Clinton c, Zack D, Curtis J, Wegener J. The Incremental Clinical Utility of a Multianalyte Assay Panel with Cell-Bound Complement Activation Products versus a Traditional ANA Testing Strategy for the Diagnosis and Treatment of SLE [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-incremental-clinical-utility-of-a-multianalyte-assay-panel-with-cell-bound-complement-activation-products-versus-a-traditional-ana-testing-strategy-for-the-diagnosis-and-treatment-of-sle/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-incremental-clinical-utility-of-a-multianalyte-assay-panel-with-cell-bound-complement-activation-products-versus-a-traditional-ana-testing-strategy-for-the-diagnosis-and-treatment-of-sle/