Session Information
Date: Saturday, November 6, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster I: Diagnosis (0323–0356)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: To determine if a serologic phenotype can be identified in SLE patients with myositis and/or ILD.
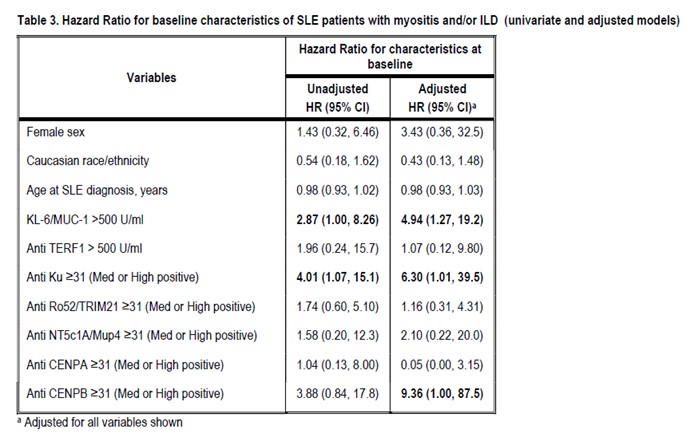
Methods: Adult SLE patients (without myositis or ILD at baseline) had annual assessments and provided bio-samples between 2000-2017. Potential new-onset myositis was identified using the SLICC Damage Index (SDI) muscle atrophy/weakness item, the SLEDAI-2K item for myositis, and annual serum creatinine kinase testing. Potential new-onset ILD was identified using the SDI pulmonary fibrosis item. Chart review confirmed cases. Randomly sampled patients from baseline visit (from 2000 onward) became a sub-cohort (N=72). Cases and sub-cohort were compared regarding baseline characteristics. Patients’ myositis-related biomarkers were assessed at baseline and one randomly selected follow-up between baseline and end of observation (date of myositis/ILD diagnosis or Dec. 31, 2017). Line immunoassay (Euroimmun AG, Luebeck, Germany) detected autoantibodies to Mi2‐α, Mi2-β, MDA5, NXP2, TIF1γ, PM/Scl75, PM/Scl100, Ku, SRP, Jo‐1, EJ, OJ, PL7, PL12, Ro52, HMGCR, NT5c1A/Mup44, CENP-A, -B, Scl70, NOR90, RNAP, and Th/To (hPOP1). An addressable laser bead immunoassay was used to detect antibodies to TERF-1. KL-6 levels were determined by ELISA (R&D Systems). Descriptive analyses and hazards ratios (HRs) were generated for myositis and/or ILD incidence, focusing on baseline serology and adjusting for demographic variables (sex, ethnicity, and age at SLE diagnosis) and positive biomarkers.
Results: The median (IQR) SLE duration at baseline was 1.8 (0.41, 5.6) years. Between 2000-2017, 14 SLE patients (12, 85.7% female) developed myositis and/or ILD over an average follow up of 9.2 years (incidence 17.6 cases per 1000 patient-years). Thirteen of these (92.9%) had at least one medium/high positive biomarker at baseline, versus 47 (65.3%) SLE patients who never developed myositis and/or ILD. The most common baseline biomarkers in patients with myositis and/or ILD were KL-6, anti-Ku, anti-Ro52. In multivariate Cox regression analyses, SLE patients were more likely to develop myositis and/or ILD if they had elevated baseline KL-6, anti-Ku positivity, or anti-CENP-B positivity. Potential limitations include the relatively low number of events.
Conclusion: In this SLE sample, KL-6, anti-Ku, and anti-CENP-B at baseline were highly associated with myositis and/or ILD risk. Ours is the first study of this serologic phenotype, identifying SLE patients most at risk of myositis/ILD.
To cite this abstract in AMA style:
Cotton T, Fritzler M, Choi M, Zheng B, Zahedi Niaki O, Grenier L, Vinet E, Pineau C, Lukusa L, Kalache F, Bernatsky S. Serologic Phenotypes Distinguish SLE Patients with Myositis And/or Interstitial Lung Disease (ILD) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/serologic-phenotypes-distinguish-sle-patients-with-myositis-and-or-interstitial-lung-disease-ild/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serologic-phenotypes-distinguish-sle-patients-with-myositis-and-or-interstitial-lung-disease-ild/