Session Information
Date: Saturday, November 6, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Impact (0225–0240)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: The availability of biosimilars has created a financial incentive to encourage non-medical switching if cheaper products are on the market. In patients with chronic inflammatory rheumatic diseases (CIRD), we have previously reported a relatively high retention rate after switching from originator etanercept to its biosimilar. However, this has been different in other studies and the reasons for non-adherence are poorly understood. Comorbidity has recently gained much attention in patients with CIRD and might be a reason for non-adherence.
The aim of this study was to analyse the effectiveness and safety of systematic non-medical switching from originator adalimumab (ADA) to ADA ABP501 biosimilar (ABP) over 6 months in patients with CIRD and to investigate the influence of comorbidities on retention rates.
Methods: Patients with CIRD on originator ADA who switched to ABP subsequently from October 2018 onwards were identified from a large routine database and then followed for 6 months. The presence of comorbidities and disease characteristics as well as measures of disease activity, physical function and changes in treatment were documented at baseline (the time of switching from originator ADA to ABP), and at months 3 and 6. Longitudinal data including information on the clinical efficacy and safety of ABP, and the reasons for discontinuation were documented.
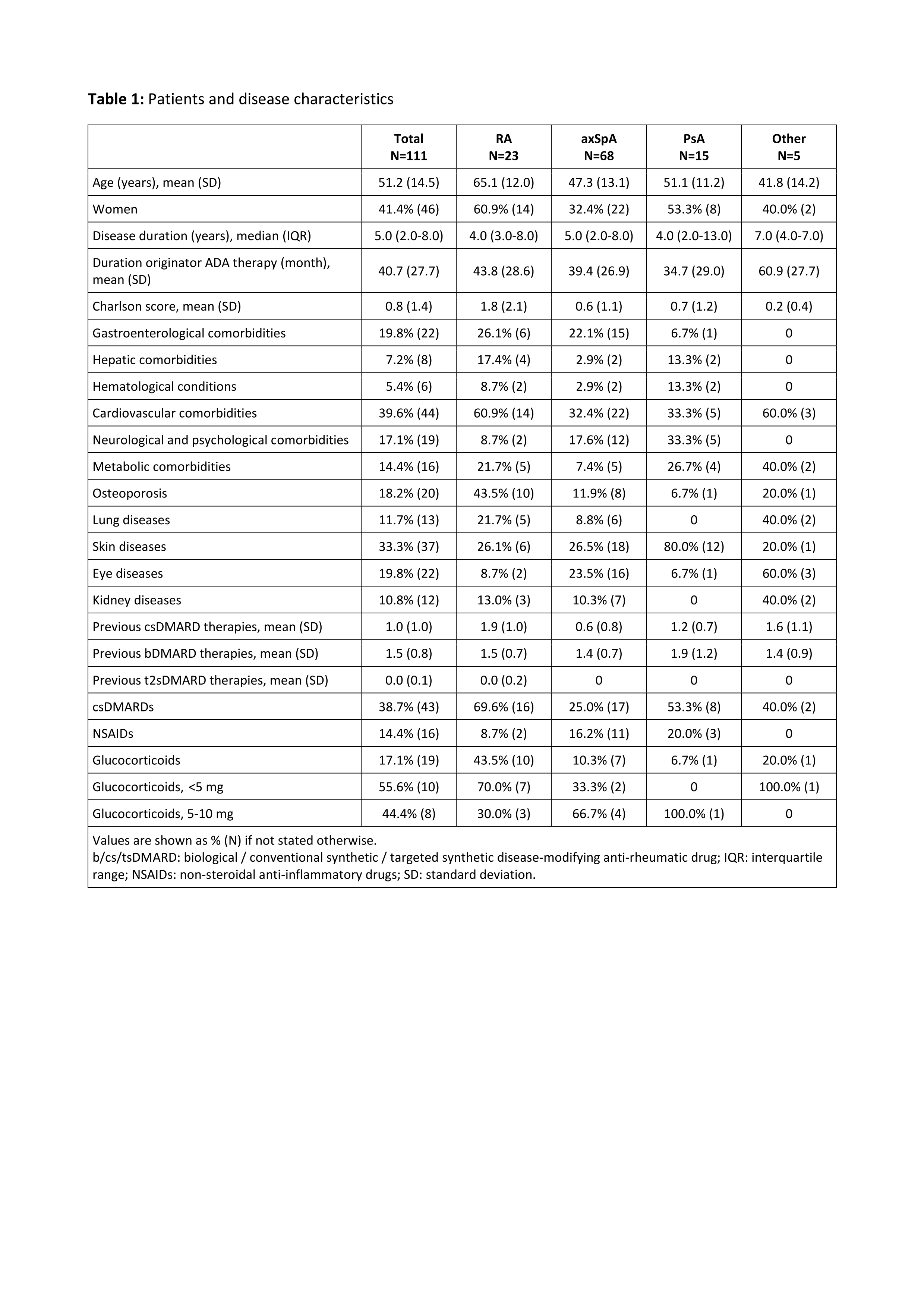
Results: A total of 111 CIRD patients on treatment with originator ADA were switched to the biosimilar ABP. This comprised 23 patients with rheumatoid arthritis (RA), 68 with axial spondyloarthritis (axSpA), and 15 with psoriatic arthritis (PsA). More than half of the patients (62%) had a Charlson comorbidity score of zero, though there were differences between disease subtypes. RA patients were comparatively older (mean age 65 years) and had the highest mean Charlson score (1.8), see Table 1.
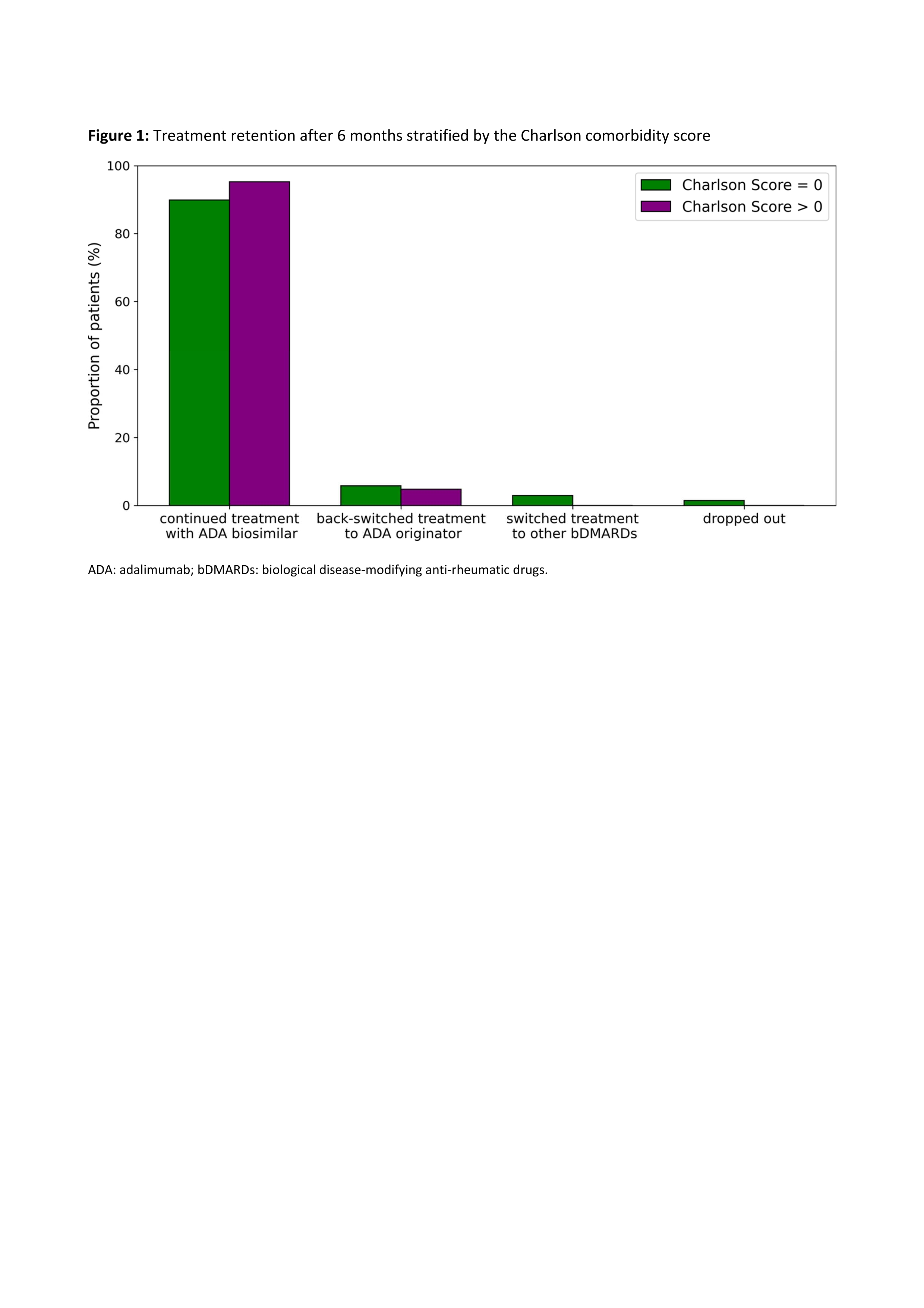
Treatment retention varied only slightly between patients with a Charlson score of zero and those with a higher score (Figure 1). In both groups, the majority of patients (90% vs 95%) continued therapy with ABP, while only a small proportion either switched back to originator ADA (6% vs 5%), switched to a different biologic (3% vs 0%), or dropped out (1% vs 0%). The main reason for back switch was the occurrence of adverse events, mostly subjective complaints, most frequently pain. Spaghetti plots show the trajectories of scores for disease activity and physical function stratified by disease subtype (Figure 2). Patients with a Charlson comorbidity score > 0 tended to have poorer scores in this regard.
Conclusion: Comorbidity had no influence on the biosimilar retention rate after 6 months in this study but the majority of patients did not have Charlson scores > 0. However, disease activity and physical function tended to be worse among CIRD patients with comorbidity. Cardiovascular disease and osteoporosis were more often present in RA patients than in axSpA or PsA patients, while neurological and psychological comorbidities were more often observed in the latter.
Figure legend on the bottom: ADA: adalimumab; bDMARDs: biological disease-modifying anti-rheumatic drugs.
Figure legend on the bottom: ASDAS: Ankylosing Spondylitis Disease Activity Score; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASFI: Bath Ankylosing Spondylitis Functional Index; CRP: C-reactive protein; DAS28: Disease Activity Score of 28 joints; HAQ: Health Assessment Questionnaire.
To cite this abstract in AMA style:
Redeker I, Moustakis S, Tsiami S, Baraliakos X, Andreica I, Buehring B, Braun J, Kiltz U. Are Comorbidities in Patients with Chronic Inflammatory Rheumatic Diseases Associated with Treatment Adherence to Biosimilars in a Non-medical Switch Scenario? [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/are-comorbidities-in-patients-with-chronic-inflammatory-rheumatic-diseases-associated-with-treatment-adherence-to-biosimilars-in-a-non-medical-switch-scenario/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/are-comorbidities-in-patients-with-chronic-inflammatory-rheumatic-diseases-associated-with-treatment-adherence-to-biosimilars-in-a-non-medical-switch-scenario/