Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 11:30AM-1:00PM
Background/Purpose: There is a high unmet need for systemic sclerosis (SSc) treatments. Ziritaxestat (ziri; GLPG1690) is an autotaxin inhibitor with a novel mechanism of action. NOVESA (NCT03798366) is a phase 2a randomized, double-blind, placebo (PBO)‑controlled trial evaluating the efficacy, safety, and tolerability of ziri for diffuse cutaneous (dc) SSc.
Methods: Patients with dcSSc were randomized (2:1) to receive oral ziri 600 mg once daily or matching PBO for 24 weeks. Protocol-defined immunosuppressive background therapies were allowed to continue unchanged if doses were stable for ≥3 months prior to ziri treatment. Eligible patients were adults with a confirmed diagnosis of dcSSc (by ACR/EULAR/Van den Hoogen/LeRoy 2013 criteria) and a modified Rodnan skin score (mRSS) >10 at screening. The primary endpoint was change from baseline mRSS at 24 weeks. Secondary endpoints were incidence of treatment-emergent adverse events (TEAEs), serious AEs and tolerability of ziri. Additional endpoints included changes in forced vital capacity (FVC), Health Assessment Questionnaire Disability Index (HAQ-DI) and Combined Response Index for Systemic Sclerosis (ACR CRISS) score. Analyses used a mixed-effects model for repeated measures. Least square (LS) mean (95% confidence interval [CI]) was calculated for the primary endpoint, descriptive statistics for other endpoints. Covariates included baseline mRSS and country.
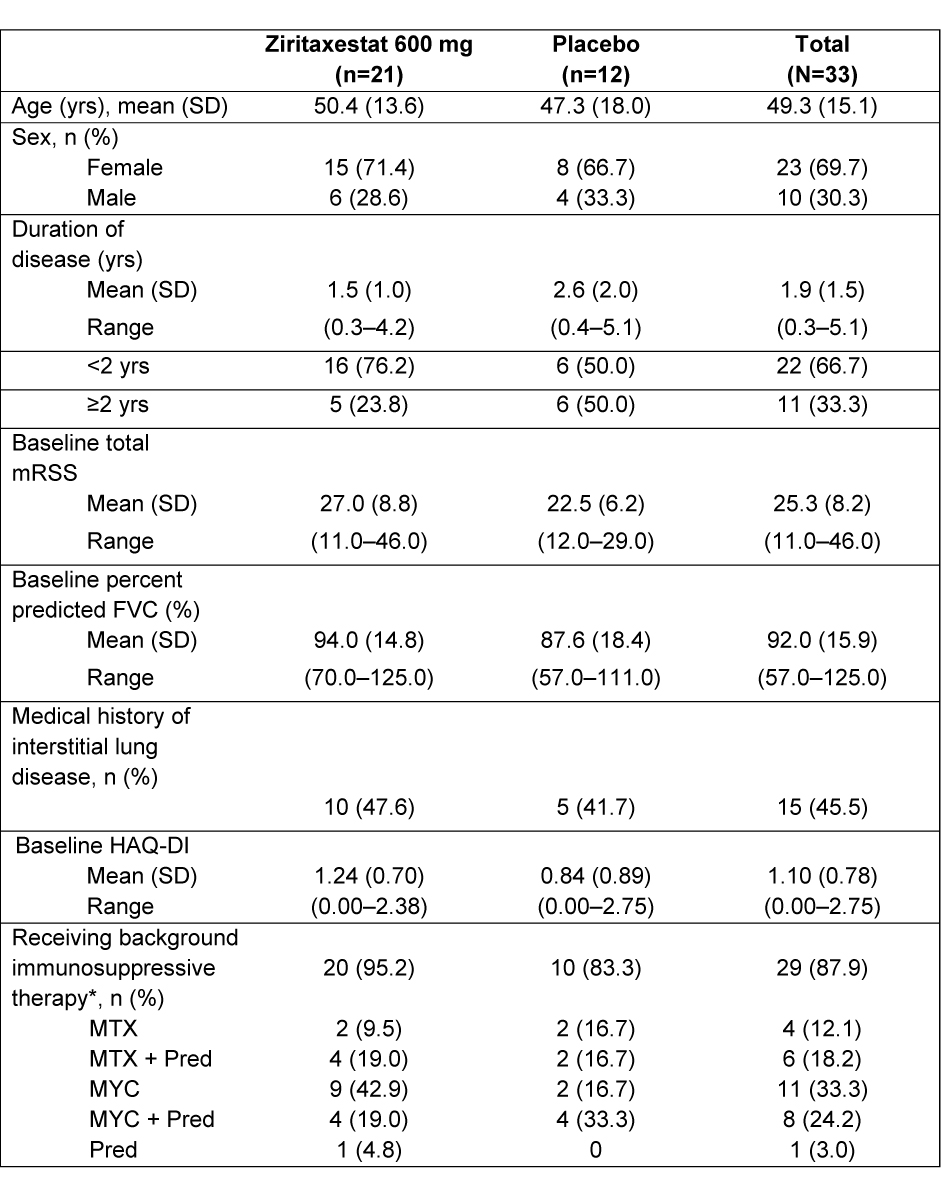
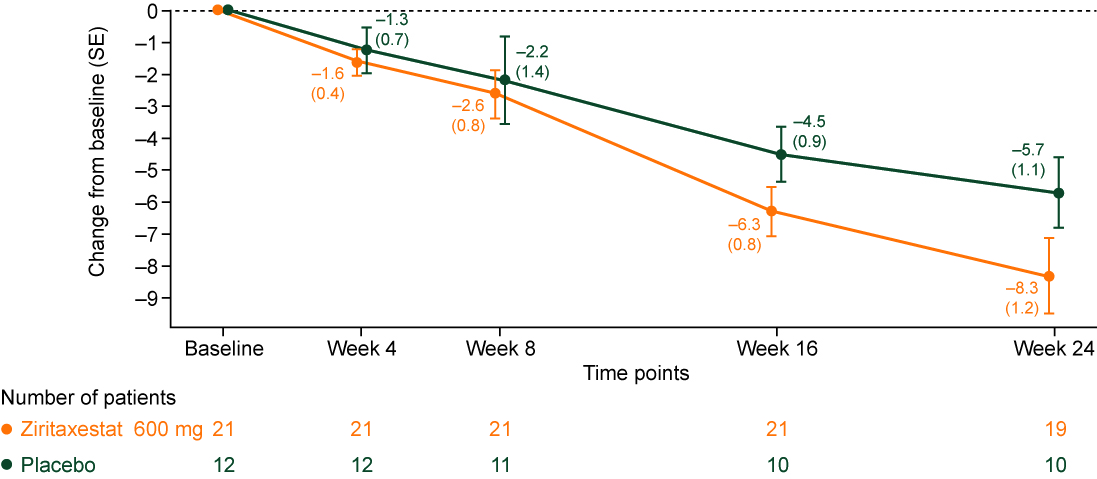
Results: Thirty-three patients with active dcSSc were randomized: 21 to ziri; 12 to PBO. The majority of patients were female (69.7%); mean (standard deviation [SD]) age was 49.3 (15.1) yrs (Table 1). In the ziri and PBO groups, respectively, mean (SD) disease duration was 1.5 (1.0) and 2.6 (2.0) yrs; mean (SD) mRSS was 27.0 (8.8) and 22.5 (6.2). 95.2% and 83.3% of patients in the ziri and PBO groups were on background immunosuppressive therapy. A statistically significant difference was observed between groups for mRSS at Wk 24: LS mean difference (95% CI) was –2.8 (–5.6, –0.1) for ziri vs PBO (p=0.0411; Figure 1). Median CRISS score was 0.97 for ziri and 0.83 for PBO at Wk 24 (0.69 in the PBO group excluding a single outlier patient with implausible Wk 24 FVC change [+1381 mL]). ACR CRISS showed likelihood of improvement (score ≥0.60 on a 0.0–1.0 scale) in 64.7% and 62.5% of patients at Wk 24 in ziri and PBO groups, respectively (57.1% of PBO patients at Wk 24 with exclusion of the FVC outlier). No changes in FVC (mL) or HAQ-DI were observed. Ziri was well tolerated; most AEs were mild or moderate; no TEAEs led to study drug discontinuation. Serious TEAEs occurred in 2 patients in the ziri group (pharyngitis and sepsis; device-related infection and sepsis) and 1 patient in the PBO group (foreign body ingestion); these were considered unrelated or unlikely related to study drug. No deaths occurred. Preliminary data show target inhibition reflected by an average reduction in circulating lysophosphatidic acid of ~80%.
Conclusion: In the small NOVESA study, ziri significantly improved mRSS vs PBO at Wk 24 and was well tolerated when administered with standard-of-care immunosuppressive therapy. Results support a possible role for the autotaxin pathway in the pathogenesis of SSc skin disease and warrant further clinical research.
*Includes MTC, MYC, and Pred
FVC, forced vital capacity; HAQ-DI, Health Assessment Questionnaire Disability Index; mRSS, modified Rodnan skin score; MTX, methotrexate; MYC, mycophenolate; Pred, prednisone; SD, standard deviation; yr, year
Changes in N are due to 1 patient being lost to follow-up and patients having the mRSS assessment outside the allowable window, due to COVID-19 restrictions limiting onsite visits
COVID-19, coronavirus disease 2019; mRSS, modified Rodnan skin score; SE, standard error
To cite this abstract in AMA style:
Khanna D, Denton C, Furst D, Mayes M, Matucci-Cerinic M, Smith V, de Vries D, Deberdt L, Stiers P, Prasad N, Ahmed S. A Phase 2a Randomized, Double-blind, Placebo-controlled Study of Ziritaxestat in Early Diffuse Cutaneous Systemic Sclerosis (NOVESA) [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-phase-2a-randomized-double-blind-placebo-controlled-study-of-ziritaxestat-in-early-diffuse-cutaneous-systemic-sclerosis-novesa/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-2a-randomized-double-blind-placebo-controlled-study-of-ziritaxestat-in-early-diffuse-cutaneous-systemic-sclerosis-novesa/