Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: The major antinuclear autoantibodies of systemic sclerosis (SSc) associate with different skin score trajectories and risk of internal organ manifestations. To elucidate molecular differences between ANA-defined subgroups, we utilised the prospective BIOPSY cohort of well-characterised SSc patients.
Methods: The prospectively collected BIOPSY cohort recruited 52 SSc patients (21 early dcSSc, 15 established dcSSc, 16 lcSSc) and 16 matched HC. 36 (69%) of the SSc patients are female. Mean disease duration in the early dcSSc cohort was 24 months (sd 12 months). ANA frequency in BIOPSY reflected the overall dcSSc population: anti-topoisomerase-1 (ATA) n= 14 (27%), anti-RNA pol III (ARA) n= 12 (23%) and other n=26 (50%). Mean baseline skin score (MRSS) for early dcSSc was 21 (sd 11.2).At a group level mRSS peak was 21.9 (11.8) at 3 months and fell to 19.1(10.5) at 12 months. For comparison, the BIOPSY cohort also included cases of established dcSSc (n=15, mean disease duration 11.3 years), lcSSc (n=16) and matched health controls (HC) (n=16). Serum biomarkers of ECM turnover and fibrosis were measured every three monthly and genome-wide transcriptomic profiling of whole skin and whole blood performed by RNA-Seq. Statistical analysis used RStudio with ANOVA, and Tukey post-hoc test. Differential gene expression used the Bioconductor limma software, with standard thresholds for significance.
Results: We found clear differences when early dcSSc patients were analysed by major ANA subset for longitudinal change in serum markers of fibrosis and in whole skin gene expression, suggesting a mechanistic basis for the distinct clinical phenotypes associated with hallmark ANAs. At baseline, there was no significant difference in soluble markers by major ANA subset. During follow-up, significant differences were observed in HA, TIMP1, and PIIINP at 6 and 12 months (p< 0.05), revealed by Tukey post-hoc analysis to reflect stable levels in ATA+ patients compared to progressively increased levels in the other subgroups.
There were 854 significantly differentially expressed genes in skin between early dcSSc and HC (Table 1) and unsupervised clustering also differentiated ARA and ATA patients with early dcSSc. 68 genes were significantly differentially expressed in skin between ATA and ARA patients (Table 2). Whilst 206 genes were differentially expressed in PBMC between early dcSSc compared with HC, only 2 genes were significantly different between ATA and ARA PBMCs (CACNG6; PLXNA2). Functional analysis suggested key biological processes distinguishing ARA and ATA skin included lipid metabolic process (PDE3B, ADIPOQ, GPAM), PPAR signaling pathway process (CD36, FABP4, PLIN1), and response to endogenous stimuli (FGF2, GPD1, FGF10)
Conclusion: We have found significant differences in skin gene expression and longitudinal change in serum markers by autoantibody specificity in dcSSc. Our findings have implications for SSc pathogenesis and support stratification by ANA subgroup in clinical studies.
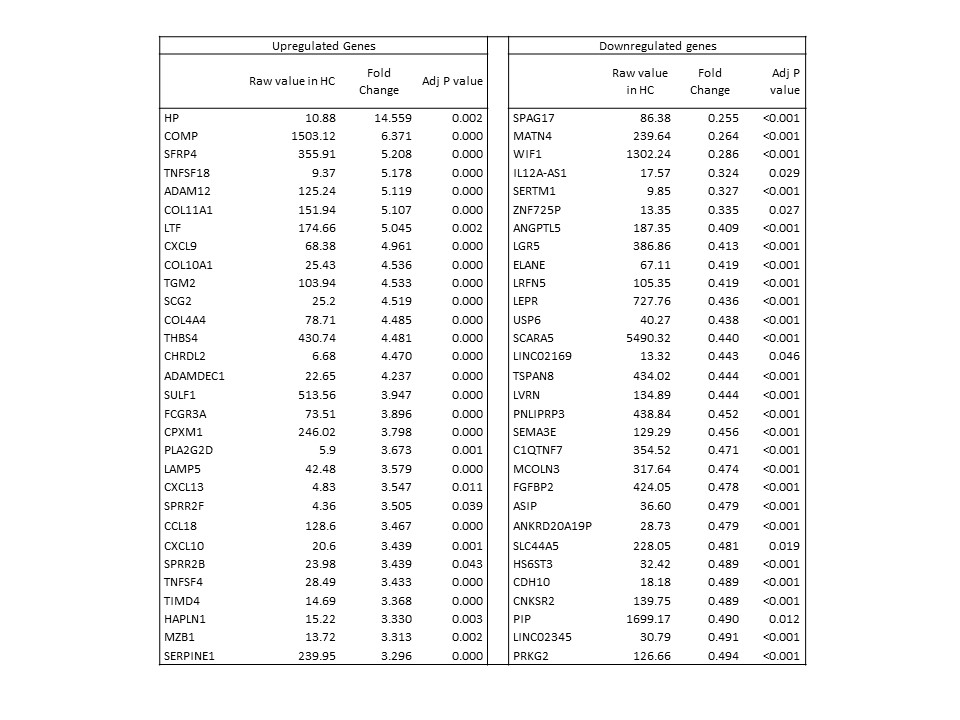 Table1: Sample genes (60 of 854) with the greatest differential expression in skin of early dcSSc compared to healthy controls with adjusted p value.
Table1: Sample genes (60 of 854) with the greatest differential expression in skin of early dcSSc compared to healthy controls with adjusted p value.
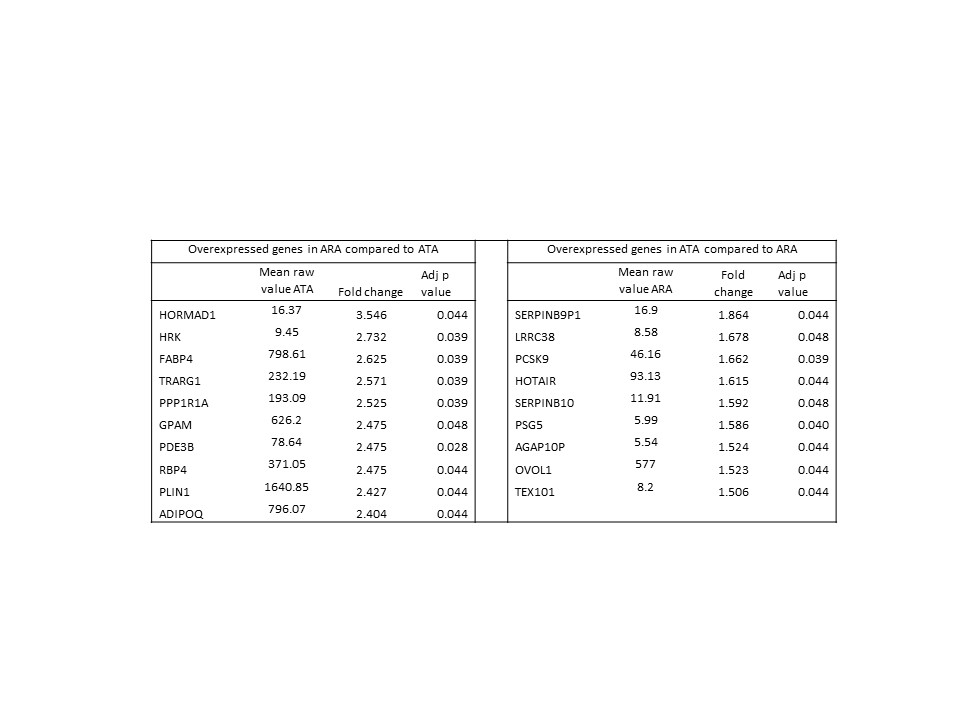 Table 2: Sample genes (n=19 of 68) differentiating ARA and ATA subtypes in early dcSSc skin
Table 2: Sample genes (n=19 of 68) differentiating ARA and ATA subtypes in early dcSSc skin
To cite this abstract in AMA style:
Clark K, Campochiaro C, Csomor E, Taylor A, Nevin K, Galwey N, Morse M, Ong V, Derrett-Smith E, Flint S, Denton C. Integrated Molecular Analysis of Systemic Sclerosis Skin and Blood Highlights Significant Differences Between Major Autoantibody Subgroups [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/integrated-molecular-analysis-of-systemic-sclerosis-skin-and-blood-highlights-significant-differences-between-major-autoantibody-subgroups/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/integrated-molecular-analysis-of-systemic-sclerosis-skin-and-blood-highlights-significant-differences-between-major-autoantibody-subgroups/
