Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Recent developments in biologic and targeted therapy have led to better control of disease activity and improved quality of life in patients with rheumatoid arthritis (RA). Many patients with RA are living longer, adding to the growing elderly population. A critical appraisal of extant data from studies on safety of these immunomodulatory therapies in the elderly can inform decision-making by patients and their physicians. We thus conducted a systematic review and meta-analysis of research studies on safety of biologics and targeted therapy in patients aged >60 years with RA.
Methods: We systematically searched Pubmed/Medline and Scopus from January 1, 1999 to June 1, 2020 to identify eligible studies that examined the safety of biologic & targeted therapies in older patients with rheumatoid arthritis. Included studies provided information on patients who received biologic (anti-TNF, IL-1, IL-6, B cell, or T cell) or targeted therapy (Janus kinase inhibitor), patients older than 60 years and a control population (younger users on biologics). Information on overall pooled rates of infections and malignancy was extracted from the studies. Meta-analysis was performed using RevMan software.
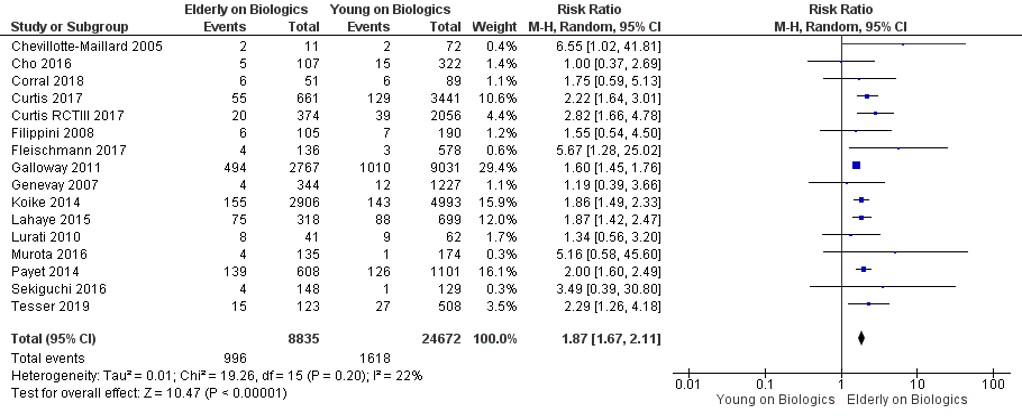
Results: We identified 823 studies; of these, 16 were included in the final analysis (12 observational studies and 4 randomized clinical trials) that comprised 8992 older users on biologics and 25,564 younger users of biologics. The pooled prevalence of infections in older and younger users of biologics was 11% and 7%, respectively, yielding a pooled random effects odds ratio of 1.87 (95% CI, 1.67–2.11). Older age was associated with a significant increase in risk of malignancy (OR, 2.32; 95% CI, 1.60–3.35) compared to younger users on biologics. The majority of the eligible studies were conducted in White population.
Conclusion: In a systematic review and meta-analysis, older patients with RA on immunomodulatory therapies were at significantly increased risk of infection and malignancy compared to younger patients on these treatments. Additional studies involving more racially and ethnically diverse cohorts are necessary to broaden our understanding of safety profile of biologics in different populations of older patients with RA. Furthermore, studies which include elderly patients on non-biologics as comparison would be necessary to better assess safety of biologics within elderly population.
To cite this abstract in AMA style:
Sood A, Al Snih S, Murthy V, Gonzalez E, Raji M. Safety of Biologic & Targeted Therapies Among Elderly Patients with Rheumatoid Arthritis: A Systematic Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/safety-of-biologic-targeted-therapies-among-elderly-patients-with-rheumatoid-arthritis-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-biologic-targeted-therapies-among-elderly-patients-with-rheumatoid-arthritis-a-systematic-review-and-meta-analysis/