Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by periods of elevated and suppressed clinical symptoms. Specific cell subsets, such as CD11c+ age-associated B cells (ABCs) have been associated with SLE pathogenesis. While TLR7 and TLR9 are thought to be prerequisites for ABC differentiation, the signaling pathways involved in driving SLE disease activity and ABC generation are still unclear.
Methods: Peripheral whole blood of healthy controls (n=18) and SLE patients with variable disease activity (n=40) were stimulated for 15 minutes with either interferon-α (IFNα), PMA and ionomycin, or Toll-like receptor (TLR) ligands for TLR4, TLR7/8 or TLR9 for phospho-protein analysis. Phenotype and phospho-protein markers were assessed by mass cytometry and cell heterogeneity was analyzed using t-SNE and manual gating. Plasma cytokines were assessed by 37-plex xMAP assays and ELISAs. SELENA-SLEDAI scores were used to correlate phenotype and functional differences with disease activity. All SLE patients met ACR classification criteria.
Results: Basal levels of pSTAT5 in CD4+ (p=0.0094) and CD8+ T cells (p=0.0289), pSTAT1 in B cells (p=0.0246), and cCASP3 in NK cells (p=0.0155) were elevated in SLE patients compared to controls. Following whole blood stimulation, a reduced fold change in pSTAT5 expression following IFNα whole blood stimulation was the primary signaling marker altered in SLE patients with high disease activity (SLEDAI≥4) compared to low disease activity (SLEDAI< 4) (p< 0.05). Most significantly, monocyte pSTAT5 IFNα-induced fold change in expression was negatively correlated with disease activity scores in SLE patients (p=0.0164) (Figure 1A). CD11c+ age-associated B cells had the strongest positive correlation with SLEDAI out of all 45 cell phenotypes assessed (p=0.0144) (Figure 1B), followed closely by HLA-DR+ CD4+ T cells (p=0.0186). A reduced fold change of pSTAT5 IFNα-induced expression in pDCs (p=0.0058), B cells (p=0.0116), and monocytes (p=0.0214) was negatively correlated with ABCs frequencies, while basal levels of pSTAT5 in pDCs, B cells, and NK cells were positively correlated with ABCs (Figure 1C, D, and E). Basal pSTAT1 levels and pSTAT1 IFNα-induced fold changes were not correlated with ABCs. Basal pSTAT5 expression in whole blood was associated with higher plasma levels of BLyS (p=0.02 x108), TNFα (p=0.02×107), IL-8 (p=0.01×103), IL-10 (p=0.00010), IL-6 (p=0.00011), SCF (p=0.0006), IL-22 (p=0.0007), and IL-31 (p=0.0018). ABCs frequencies were also strongly correlated with TNF-β (p=0.0019) plasma levels in SLE.
Conclusion: ABCs are highly correlated with disease activity and exhausted pSTAT5 IFNα-induced signaling in pDCs and other antigen-presenting cells suggesting an important role for this pathway in lupus pathogenesis and ABC differentiation.
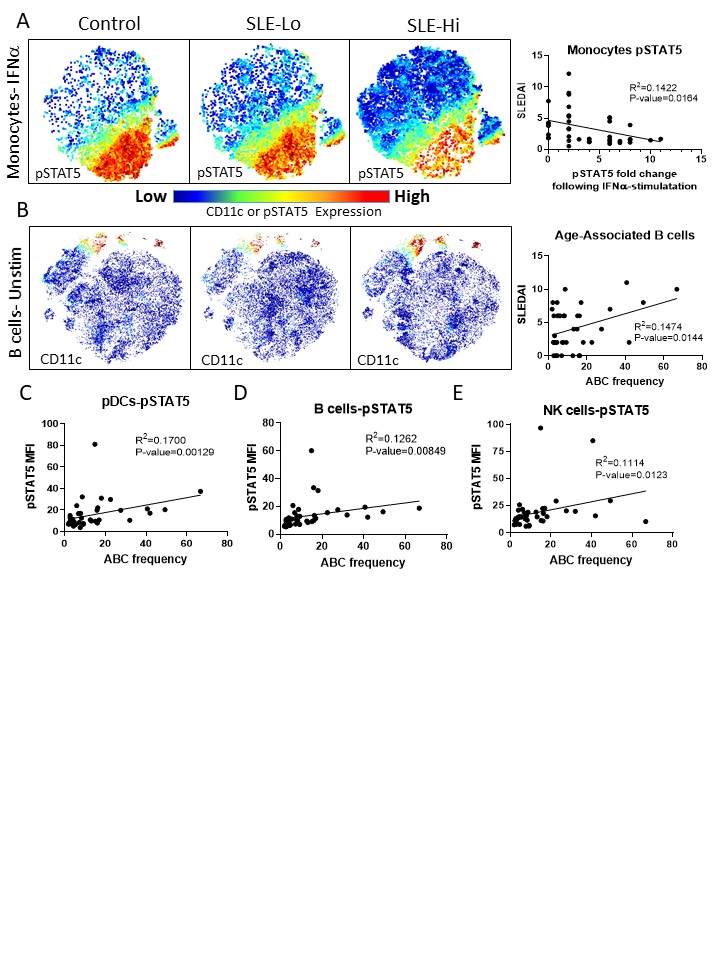 Figure 1. pSTAT5-IFNa signaling pathways are associated with age-associated B cells (ABCs). Whole blood of 58 subjects, either controls or SLE patients with variable disease activity, were used for phenotype analysis by mass cytometry. T-distributed stochastic neighbor embedding (tSNE) was used to visualize and cluster cells that were most similar using 28 surface markers. B cells or monocytes were gating and a second tSNE visualization was rendered to cluster and differentiate B cells or monocytes (A, B). pSTAT5 signaling was strongly reduced in monocytes following IFNa stimulation suggesting exhaustion of this pathway from persistent activation, and this was positively correlated with SLEDAI (A). CD11c+ ABC frequencies were higher in SLE patients, and correlated with lupus disease activity (B). Basal levels of pSTAT5 in pDCs (C), B cells (D), and NK cells (E) were correlated with ABC frequencies.
Figure 1. pSTAT5-IFNa signaling pathways are associated with age-associated B cells (ABCs). Whole blood of 58 subjects, either controls or SLE patients with variable disease activity, were used for phenotype analysis by mass cytometry. T-distributed stochastic neighbor embedding (tSNE) was used to visualize and cluster cells that were most similar using 28 surface markers. B cells or monocytes were gating and a second tSNE visualization was rendered to cluster and differentiate B cells or monocytes (A, B). pSTAT5 signaling was strongly reduced in monocytes following IFNa stimulation suggesting exhaustion of this pathway from persistent activation, and this was positively correlated with SLEDAI (A). CD11c+ ABC frequencies were higher in SLE patients, and correlated with lupus disease activity (B). Basal levels of pSTAT5 in pDCs (C), B cells (D), and NK cells (E) were correlated with ABC frequencies.
To cite this abstract in AMA style:
Slight-Webb S, Smith M, Thomas K, Macwana S, Maecker H, Utz P, James J, Guthridge J. Exhausted pSTAT5-IFNα Signaling Pathways in SLE Patients Are Correlated with Age-associated B Cells and Disease Activity [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/exhausted-pstat5-ifn%ce%b1-signaling-pathways-in-sle-patients-are-correlated-with-age-associated-b-cells-and-disease-activity/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exhausted-pstat5-ifn%ce%b1-signaling-pathways-in-sle-patients-are-correlated-with-age-associated-b-cells-and-disease-activity/
