Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease characterized by the production of autoantibodies. For this reason, anti-B cell therapy seems to be a promising approach. However, B cell depletion with Rituximab, an anti-CD20 monoclonal antibody (mAb), failed to meet the primary endpoint in large clinical trials, possibly because antibody-producing plasma blasts/cells express low levels of CD20. Therefore, strategies that specifically target the depletion of plasma blasts/cells may be effective in treating SLE. Moreover, Natural killer (NK) cells are dysfunctional in patients with SLE, and restoration of NK cell immunity has been shown to promote the destruction of plasma cells in other diseases such as multiple myeloma. Herein, we hypothesize that alterations in NK cells contribute to the pathophysiology of SLE and the restoration of NK cell immunity may promote the killing of autoantibody-producing plasma blasts/cells.
Methods: The extracellular phenotype of cryopreserved peripheral blood mononuclear cells (PBMC) from 31 SLE patients and 31 gender-, age- and ethnicity-matched healthy controls (HC) was examined by mass cytometry (CyTOF). Functional assays (cytolytic enzyme expression, cytokine production, degranulation, depletion assay) and signaling (calcium influx, phosphoprotein measurements) were examined by flow cytometry. NK cells were pre-treated with anti-SLAMF7 (Elotuzumab) or anti-CD38 (Daratumumab) mAb and were then co-cultured with B cells, to evaluate the killing of plasma blasts by activated NK cells.
Results: Our data shows that SLE NK cells are reduced in number and in function (IFNγ and TNFα production after activation with cytokines). SLE NK cells exhibit phenotypic alterations with respect to HC: (1) SLE NK cells express increased levels of CD38 and (2) fail to upregulate SLAMF7 upon activation with exogenous cytokines (Figure 1). In addition, circulating SLE plasma blasts are increased in the peripheral blood compared to HC. Moreover, plasma blasts express very high levels of CD38 and SLAMF7 (Figure 2). The engagement of SLAMF7 and CD38 with mAb partially restores the function of SLE NK cells (production of IFNγ and TNFα, degranulation). Furthermore, commercially available mAb directed against SLAMF7 (Elotuzumab) and CD38 (Daratumumab) promote the immunogenicity of NK cells to specifically kill plasma blasts (Figure 3).
Conclusion: We showed that SLAMF7 and CD38 are aberrantly expressed or regulated on the surface of NK cells of SLE patients. The engagement of SLAMF7 or CD38 with mAb restores the function of SLE NK cells and promotes the killing of plasma blasts by NK cells. Our data indicates that targeting these receptors may represent future therapeutic options in SLE to eliminate autoantibody-producing cells.
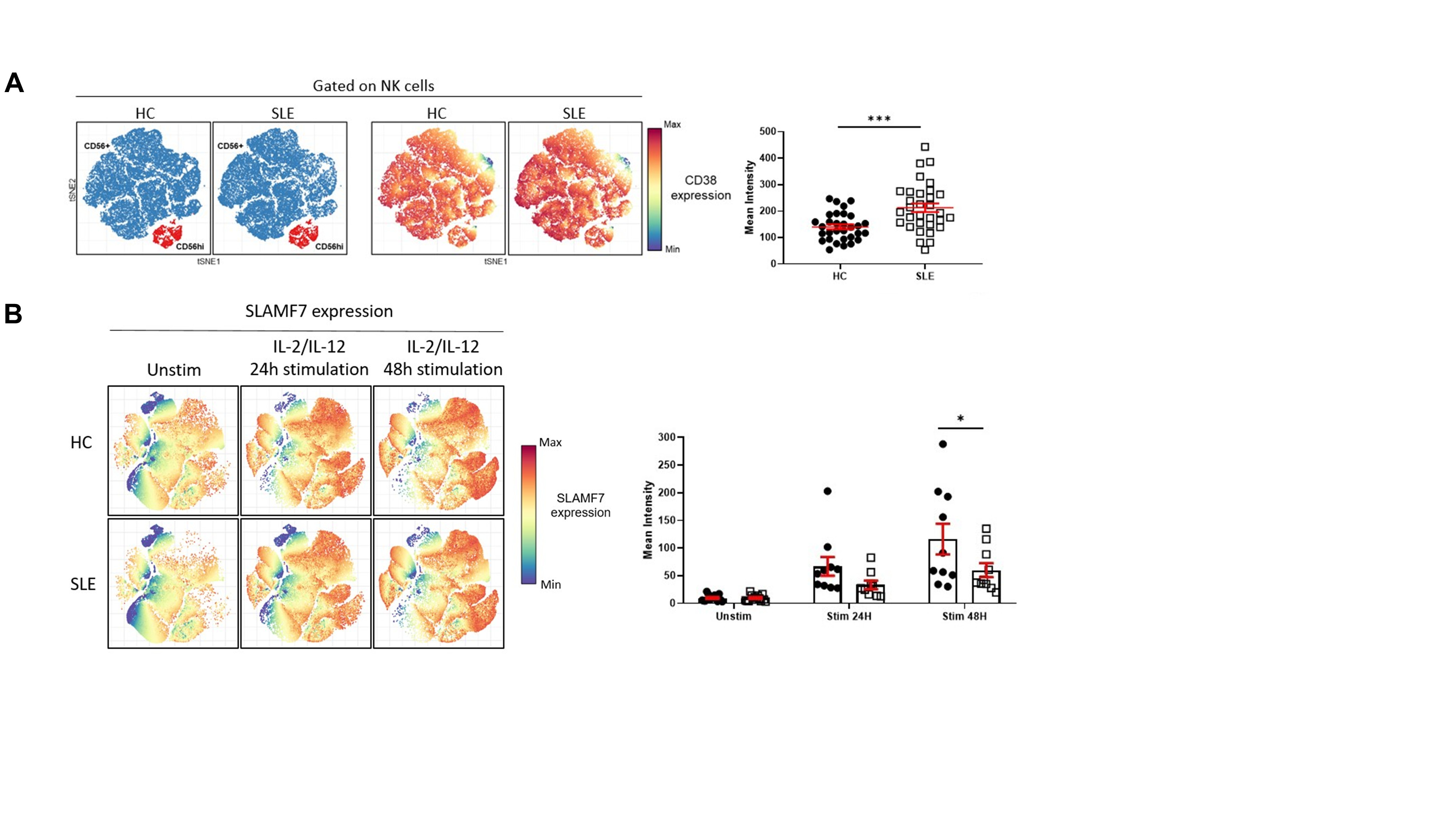 Figure 1. CD38 and SLAMF7 expression is increased on NK cells from SLE patients A. tSNE representation of NK cells distribution and CD38 expression (blue: CD56+; red: CD56hi) in healthy controls (HC) and SLE patients (left panel). Mean intensity of CD38 on total NK cells is significantly increased in SLE patients (right panel, shown as mean±SEM, Welch’s t test , p < 0.001). B. tSNE representation of SLAMF7 expression in NK cells in HC and SLE patients following 24H and 48H cytokine stimulation (left panel). SLE NK cells lack the ability of increasing the expression of SLAMF7 following cytokine stimulation (right panel, shown as mean±SEM, Two way ANOVA, Sidak’s multiple comparison, p < 0.05).
Figure 1. CD38 and SLAMF7 expression is increased on NK cells from SLE patients A. tSNE representation of NK cells distribution and CD38 expression (blue: CD56+; red: CD56hi) in healthy controls (HC) and SLE patients (left panel). Mean intensity of CD38 on total NK cells is significantly increased in SLE patients (right panel, shown as mean±SEM, Welch’s t test , p < 0.001). B. tSNE representation of SLAMF7 expression in NK cells in HC and SLE patients following 24H and 48H cytokine stimulation (left panel). SLE NK cells lack the ability of increasing the expression of SLAMF7 following cytokine stimulation (right panel, shown as mean±SEM, Two way ANOVA, Sidak’s multiple comparison, p < 0.05).
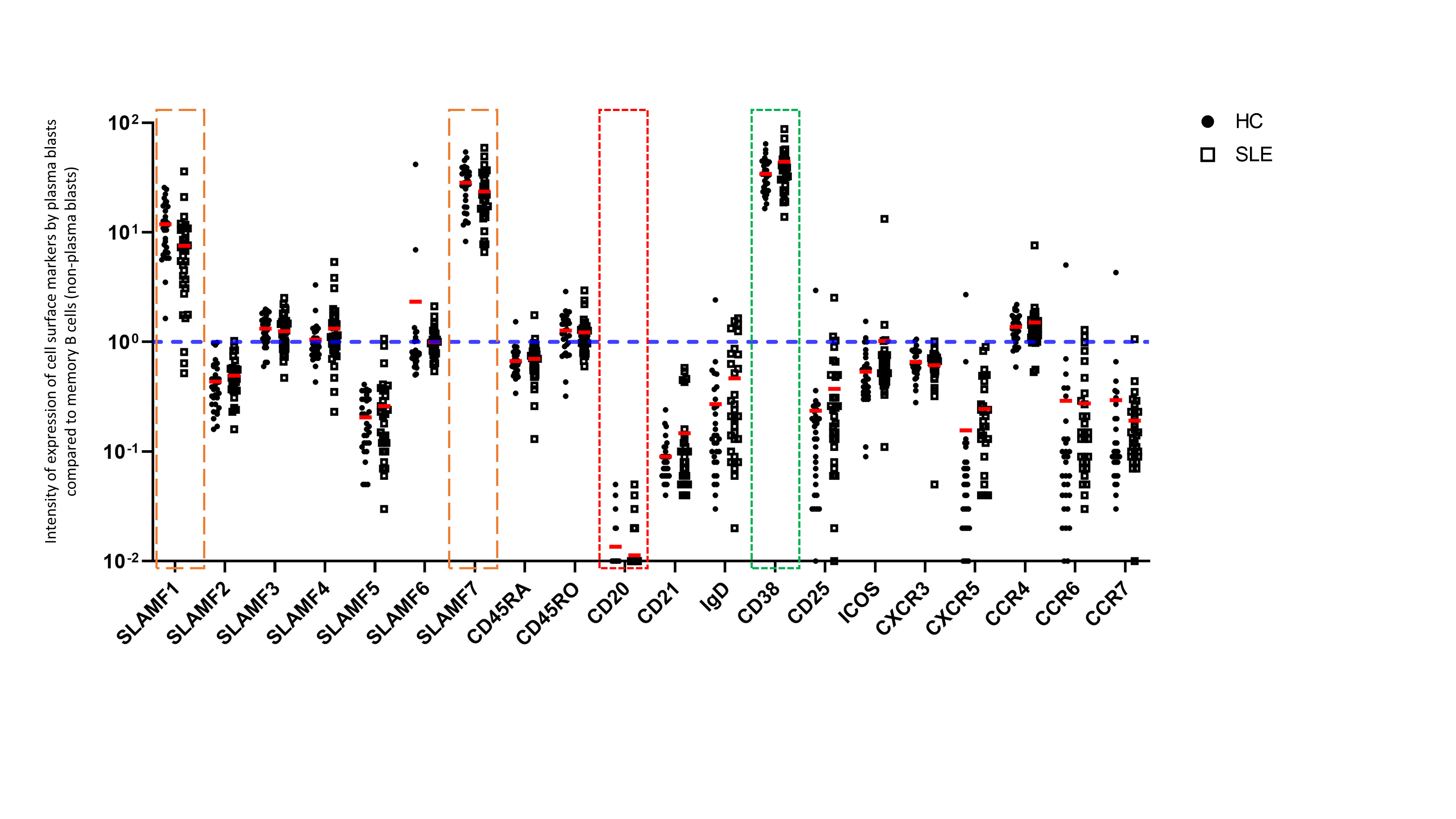 Figure 2. Expression of cell surface markers by plasma blasts from the peripheral blood of SLE patients The intensity of expression of cell surface markers is represented as an expression ratio of circulating plasma blasts (CD19+ CD27+ CD38+ CD20-) on memory B cells (non-plasma blasts) (CD19+ CD27+ CD38-). Accordingly, CD38 represents a positive control (in green) and CD20 represents a negative control (in red). These data show that SLAMF1 and SLAMF7 (in orange) are strongly expressed by plasma blasts compared to other populations of memory B cells. No difference was observed in the expression of these receptors between HC and SLE patients.
Figure 2. Expression of cell surface markers by plasma blasts from the peripheral blood of SLE patients The intensity of expression of cell surface markers is represented as an expression ratio of circulating plasma blasts (CD19+ CD27+ CD38+ CD20-) on memory B cells (non-plasma blasts) (CD19+ CD27+ CD38-). Accordingly, CD38 represents a positive control (in green) and CD20 represents a negative control (in red). These data show that SLAMF1 and SLAMF7 (in orange) are strongly expressed by plasma blasts compared to other populations of memory B cells. No difference was observed in the expression of these receptors between HC and SLE patients.
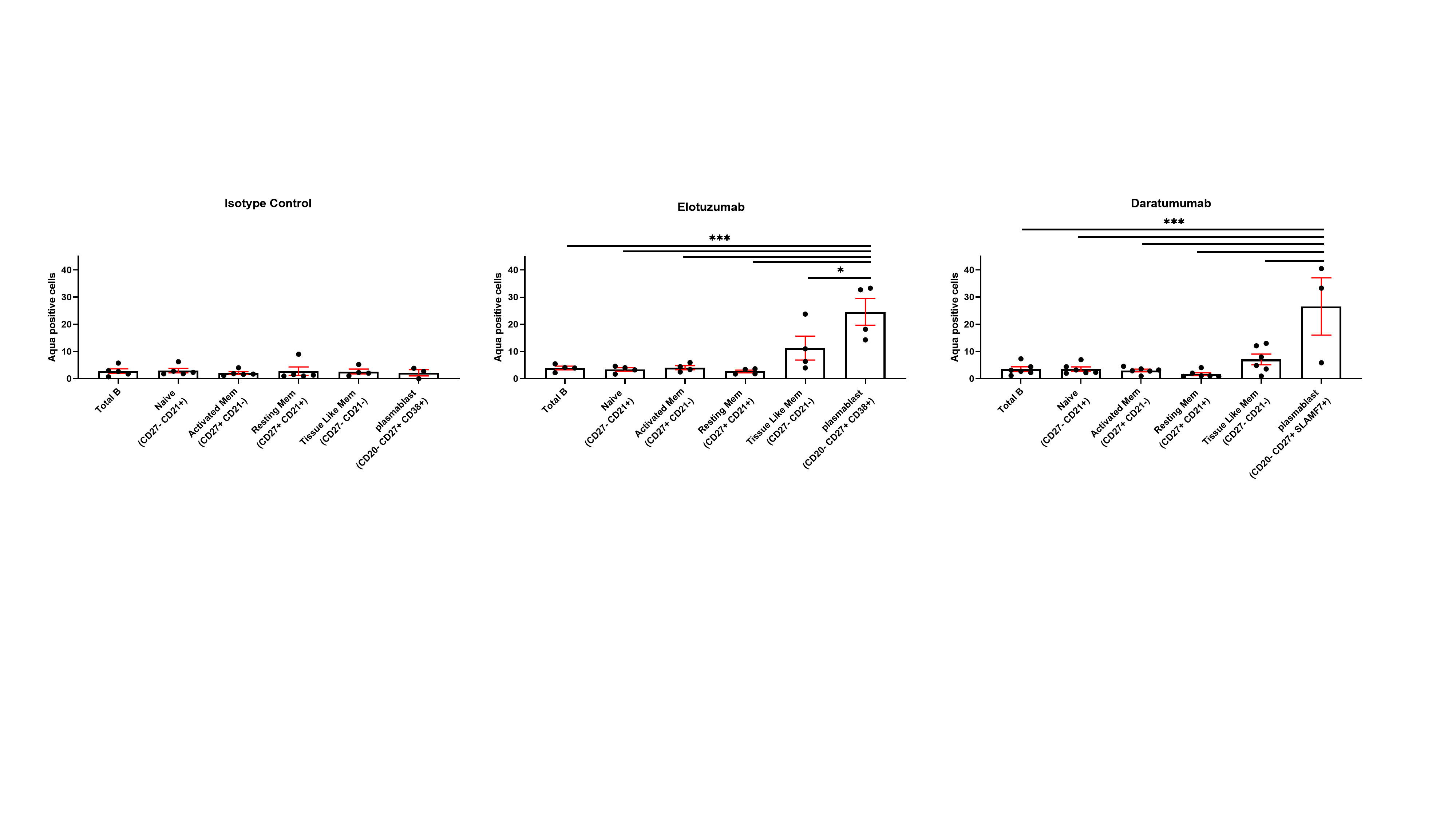 Figure 3. NK stimulation with Elotuzumab and Daratumumab promotes plasmablast killing. The engagement of NK cells with Elotuzumab and Daratumumab predominantly promotes the killing of plasmablasts compared to other B cell subpopulations (One way ANOVA Sidak’s multiple comparison, *p < 0.05, **p < 0.005,***p < 0.0001).
Figure 3. NK stimulation with Elotuzumab and Daratumumab promotes plasmablast killing. The engagement of NK cells with Elotuzumab and Daratumumab predominantly promotes the killing of plasmablasts compared to other B cell subpopulations (One way ANOVA Sidak’s multiple comparison, *p < 0.05, **p < 0.005,***p < 0.0001).
To cite this abstract in AMA style:
Humbel M, Bellanger F, Fenwick C, Horisberger A, Ribi C, Comte D. SLAMF7 and CD38 on NK Cells Represent Potential New Therapeutic Targets for Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/slamf7-and-cd38-on-nk-cells-represent-potential-new-therapeutic-targets-for-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/slamf7-and-cd38-on-nk-cells-represent-potential-new-therapeutic-targets-for-systemic-lupus-erythematosus/
