Session Information
Date: Tuesday, November 12, 2019
Title: 5T090: Osteoarthritis – Clinical II: Novel Therapies (2756–2761)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Results from the 5-year Phase II FORWARD study showed significant dose-dependent modification of total femorotibial joint (TFTJ) cartilage thickness change with sprifermin at 2 and 3 years, by quantitative MRI. Total WOMAC scores improved by ~50% in all treatment groups, including placebo (PBO). Selection of a patient (pt) subgroup with higher pain scores and lower joint space width (JSW) at baseline (BL), to identify pts who are at higher risk of further structural and symptomatic progression, may facilitate better WOMAC discrimination.
We undertook a post-hoc analysis to evaluate cartilage thickness changes and symptomatic outcomes in an ‘at risk’ subgroup (BL medial or lateral minimum [m]JSW 1.5–3.5 mm and BL WOMAC pain score of 40–90 on a scale of 0-100).
Methods: Pts in FORWARD were randomized 1:1:1:1:1 to: sprifermin 100 µg every 6 months (q6mo); 100 µg q12mo; 30 µg q6mo; 30 µg q12mo; and PBO. The treatment period was 2 years, with an extended follow up of 3 years. Post-hoc analysis was conducted in the ‘at risk’ subgroup.
Changes over time are presented using descriptive statistics and CIs. Treatment benefits were estimated using a repeated measures model, controlling for BL, treatment, time, pooled country and treatment by time interaction. Linear dose-effect trend tests were performed exploratively at each timepoint. Confidence intervals (CIs) for treatment benefit were adjusted for multiplicity of treatments using Dunnett adjustment.
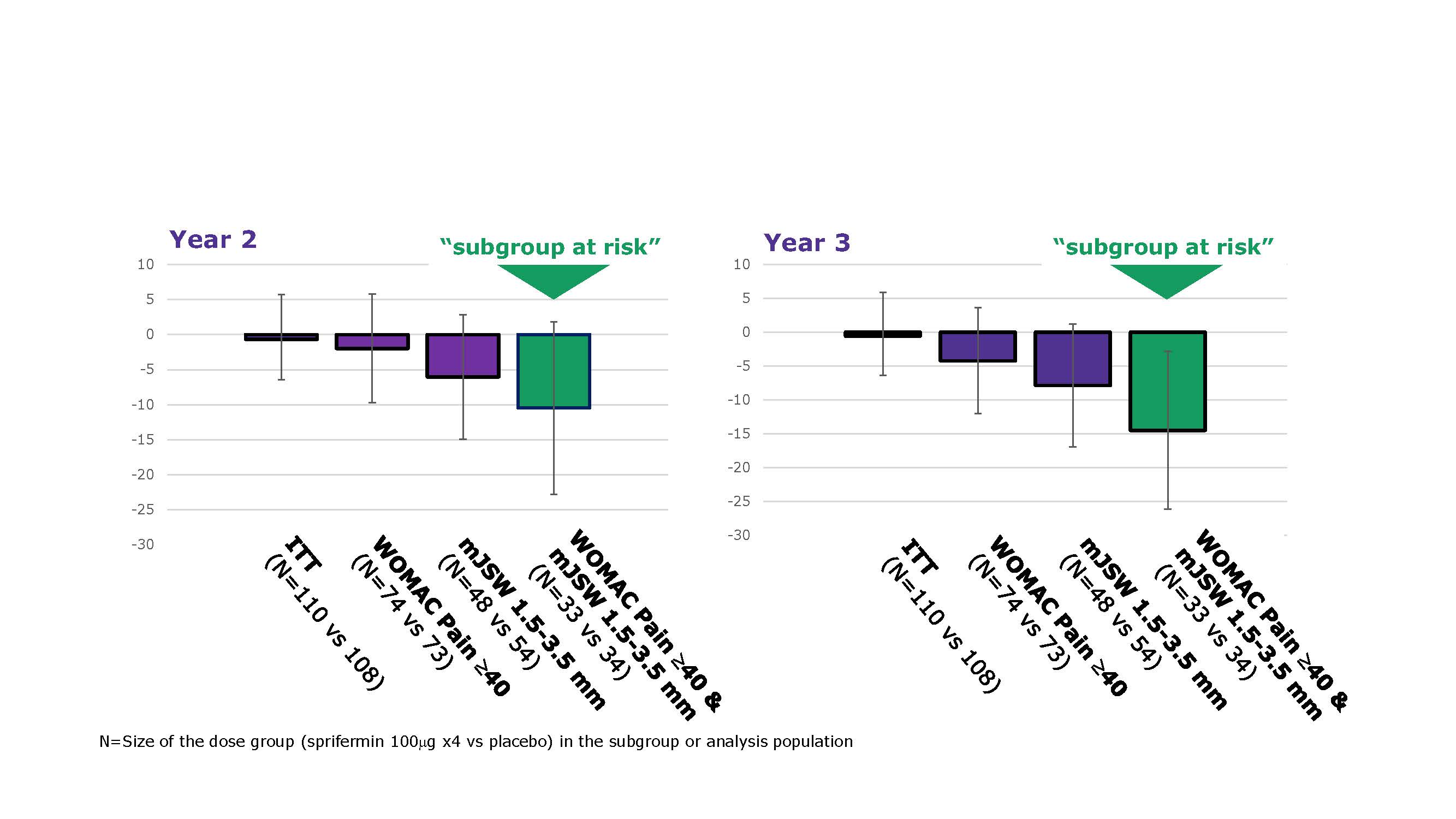
Results: 161/549 (29%) pts met criteria for the ‘at risk’ subgroup. In this subgroup, BL characteristics were quite balanced between treatment arms. Pts in the PBO arm had more cartilage loss at 2 and 3 years vs the modified intent-to-treat (mITT) PBO arm (mean change from BL in mm [SD]: Year 2 -0.05 [0.10] vs -0.02 [0.07]; Year 3 -0.07 [0.09] vs -0.05 [0.07]), but TFTJ net cartilage modification with sprifermin 100 µg q6mo vs PBO was similar (adjusted mean difference from PBO [95% CI] in the ‘at risk’ subgroup vs the mITT group: Year 2 0.06 [0.01, 0.11] vs 0.05 [0.02, 0.08]; Year 3 0.05 [-0.01, 0.12] vs 0.05 [0.02, 0.09]). At Year 3 (18 mos after last injection), the mean difference [95% CI] in WOMAC pain score for sprifermin 100 µg q6mo vs PBO in the ‘at risk’ subgroup was -8.75 [-22.42, 4.92] vs 0.97 [-6.22, 8.16] for the intent-to-treat (ITT) population (Fig 1). The exploratory dose-effect trend test using the adjusted values had a nominal p-value of < 0.05 (Table 1). Having both medial or lateral mJSW 1.5–3.5 mm AND pain score ≥40 at BL led to greater differentiation in WOMAC pain scores for sprifermin 100 µg q6mo vs PBO than having either BL characteristic alone (Fig 2).
Conclusion: Despite substantial structural and symptomatic progression in the ‘at risk’ subgroup, structural improvement with sprifermin was maintained, and WOMAC score improvements vs PBO increased over time and were significant at Year 3. This supports further investigation of sprifermin as a potential disease-modifying osteoarthritis drug in a targeted population where structural improvement may translate into symptomatic benefit vs PBO within a reasonable timeframe.

WOMAC pain SAR & ITT Figure 1 LLv5
To cite this abstract in AMA style:
Guehring H, Kraines J, Moreau F, Daelken B, Ladel C, Wirth W, Conaghan P, Eckstein F, Hochberg M. Cartilage Thickness Modification with Sprifermin in Knee Osteoarthritis Patients Translates into Symptomatic Improvement over Placebo in Patients at Risk of Further Structural and Symptomatic Progression: Post-Hoc Analysis of a Phase II Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/cartilage-thickness-modification-with-sprifermin-in-knee-osteoarthritis-patients-translates-into-symptomatic-improvement-over-placebo-in-patients-at-risk-of-further-structural-and-symptomatic-progress/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cartilage-thickness-modification-with-sprifermin-in-knee-osteoarthritis-patients-translates-into-symptomatic-improvement-over-placebo-in-patients-at-risk-of-further-structural-and-symptomatic-progress/